Abstract
In the Xenopus embryo, blastomeres are joined by gap junctions that allow the movement of small molecules between neighboring cells. Previous studies using Lucifer yellow (LY) have reported asymmetries in the patterns of junctional communication suggesting involvement in dorso-ventral patterning. To explore that relationship, we systematically compared the transfer of LY and neurobiotin in embryos containing 16–128 cells. In all cases, the junction-permeable tracer was coinjected with a fluorescent dextran that cannot pass through gap junctions. Surprisingly, while LY appeared to transfer in whole-mount embryos, in no case did we observe junctional transfer of LY in fixed and sectioned embryos. The lack of correspondence between data obtained from whole-mounts and from sections results from two synergistic effects. First, uninjected blastomeres in whole-mounts reflect and scatter light originating from the intensely fluorescent injected cell, creating a diffuse background interpretable as dye transfer. Second, the heavier pigmentation in ventral blastomeres masks this scattered signal, giving the impression of an asymmetry in communication. Thus, inspection of whole-mount embryos is an unreliable method for the assessment of dye transfer between embryonic blastomeres. A rigorous and unambiguous demonstration of gap junctional intercellular communication demands both the coinjection of permeant and impermeant tracers followed by the examination of sectioned specimens. Whereas LY transfer was never observed, neurobiotin was consistently transferred in both ventral and dorsal aspects of the embryo, with no apparent asymmetry. Ventralization of embryos by UV irradiation and dorsalization by Xwnt-8 did not alter the patterns of communication. Thus, our results are not compatible with current models for a role of gap junctional communication in dorso-ventral patterning.
Keywords: Gap junctions, dye transfer, dorso-ventral axis, Xwnt-8, UV irradiation, Lucifer yellow, neurobiotin, cytoplasmic bridges, Xenopus
Introduction
Gap junctional intercellular communication (GJIC) in early development has been most extensively explored in the amphibian Xenopus laevis using fluorescein (M r = 332) and Lucifer yellow (LY; M r = 457) as reporter molecules (Guthrie 1984; Warner et al. 1984; Cardellini et al. 1988). A marked asymmetry of dye movement between presumptive dorsal and ventral sides of the animal pole was noted as early as the 16-cell stage (Guthrie et al. 1988). Later studies examined the effect of conditions that alter dorso-ventral patterning on dye transfer to further implicate GJIC in dorsal development (Nagajski et al. 1989; Olson et al. 1991; Olson and Moon 1992; Guger and Gumbiner 1995; Krufka et al. 1998). Experimental conditions that dorsalize embryonic development, such as lithium treatment (Nagajski et al. 1989) and injections of RNA coding for wnt-1 (Olson et al. 1991; Olson and Moon 1992), activin B (Olson and Moon 1992), and β-catenin (Guger and Gumbiner 1995), resulted in the increased intercellular transfer of LY between presumptive ventral blastomeres in 32-cell stage embryos. Conversely, manipulations that ventralize embryonic development, such as UV irradiation (Nagajski et al. 1989) and depletion of β-catenin (Krufka et al. 1998), result in decreased dye transfer between the presumptive dorsal blastomeres of 32-cell stage embryos. Together, these findings suggest a requirement for GJIC, as measured by LY transfer, in early patterning events.
For the most part, the previous studies have used whole-mounts of unsectioned embryos after injection of LY for the assessment of dye transfer. Whereas the whole-mount method is widely accepted, it is difficult to construct an accurate map of the patterns of dye movement. Therefore we analyzed the patterns of GJIC during early Xenopus embryonic development using fixed, sectioned embryos injected with a variety of small (<1,000 D) tracers. Three tracers were fluorescent (LY, Alexa 350, Alexa 488), while a fourth, neurobiotin, was localized with a fluorescent avidin or streptavidin reagent. To insure that any apparent cell–cell transfer was due to GJIC, we coinjected 10-kD fixable fluorescent dextrans that were too large to pass through junctional channels. We followed cleavage patterns of Xenopus embryo blastomeres and compared the fluorescence of dyes in whole-mount embryos with dye transfer observed in paraffin sections. Surprisingly, we were unable to demonstrate any intercellular transfer of LY without accompanying fluorescent dextran within the time scales generally used for these studies. We found that the fluorescent signal emanating from an injected dorsal blastomere can reflect and be scattered within the whole-mount embryo, creating the impression of GJIC. In addition, the higher levels of pigment in the ventral blastomeres masked fluorescence, creating the impression of lower levels of GJIC on the ventral-animal pole of whole-mount Xenopus embryos. We also found evidence for the presence of patent intercellular cytoplasmic bridges beyond the 8-cell stage. In contrast to LY, neurobiotin was widely transferred among all animal blastomeres up to the 128-cell stage and among vegetal blastomeres up to stage 8. However, we found no obvious asymmetry in the pattern of neurobiotin transfer. Most importantly, neurobiotin intercellular transfer was not detectably affected by conditions that altered dorso-ventral patterning such as Wnt expression or UV treatment, conditions reported to change LY coupling in earlier studies.
We conclude that proper analysis of dye transfer in the Xenopus embryo cannot be performed on whole-mounts and requires both the coinjection of dextran dye complexes together with the low molecular weight tracers and sectioning. In addition, while we observed extensive GJIC at early stages, we found no evidence for asymmetry in its pattern that could plausibly contribute to determination of the embryonic axis. Thus, our results are not compatible with current models for the role of GJIC in dorso-ventral patterning.
Materials and Methods
General
Assays using Xenopus oocytes and embryos were all done at a constant temperature of 18°C. Fertilized Xenopus eggs and embryos were kept in 0.1 MMR (Peng 1991) and were transferred into 0.1 MMR, 5% Ficoll (pH 7.4) before injections. The volume of dye (tracers and fluorescent conjugates) injected varied, depending on the size of the injected blastomere. Typically, ∼2 nl at the 16-cell embryo, 0.5–2 nl at the 32-cell embryo, 0.5–1 nl at the 64-cell embryo and 0.1–0.5 nl at the 128-cell embryo. For each experiment, a cohort of the injected embryos was allowed to continue development to insure that the dye injection did not interfere with subsequent cell cleavages (see Fig. S6, A–D, control panels).
Tracers and Fluorescent Conjugates
The following traces and conjugates were used: Neurobiotin, mol wt = 322.85 (Vector Laboratories); LY, lithium salt, mol wt = 457.24 (Molecular Probes); Alexa 350 hydrazide, sodium salt, mol wt = 349.29 (Molecular Probes); Alexa 488 hydrazide, sodium salt, mol wt = 570.48 (Molecular Probes); dextran-fluorescein, lysine fixable, mol wt = 10,000 (Molecular Probes); dextran-rhodamine, lysine fixable, mol wt = 10,000 (Molecular Probes); dextran-biotin, lysine fixable, mol wt = 10,000 (Molecular Probes); avidin-rhodamine conjugate (Pierce Chemical Co.); and Streptavidin Alexa 488 conjugate (Molecular Probes).
Tracer Mixtures
Free, unbound fluorescent molecules were removed from the dextran tracer solutions on Biomax-5K, 15 ml, Millipore filters. The following mixtures were prepared: 7.5% neurobiotin, 2% dextran-rhodamine, and 50 mM Hepes, pH 7.8; 7.5% neurobiotin, 2% dextran-fluorescein, and 50 mM Hepes, pH 7.8; 2% LY, 2% dextran-rhodamine, and 50 mM Hepes, pH 7.8; 2% Alexa 350, 2% dextran rhodamine, and 50 mM Hepes, pH 7.8; 2% Alexa 488, 2% dextran-rhodamine, and 50 mM Hepes, pH 7.8; and 2% dextran-biotin, 2% dextran-rhodamine, and 50 mM Hepes, pH 7.8.
Histology
10 min after dye injection, embryos were fixed for 2–6 h in 4% formaldehyde (EM grade; Electron Microscopy Sciences). Analysis of whole-mount embryos was done either during or at the end of the fixation time. This early analysis was aimed to make sure that all injections were successfully done, that blastomeres of all embryos were not damaged, and that no leaks resulted either during injection or fixation. This also allowed whole-mount analysis of transfer of LY, Alexa 350, and Alexa 488. After fixation, embryos were embedded in paraffin and ∼70, 12 μM serial sections were cut through each embryo.
Sections of embryos injected with mixtures containing neurobiotin and dextran biotin were deparaffinized through graded steps of xylene and alcohol and immediately processed for the detection of biotin. Sections of embryos injected with mixtures containing LY, Alexa 355 and Alexa 488 were deparaffinized and then were either mounted in Gurr (a xylene-based medium) or were gradually passed through alcohol to PBS and mounted in Vectashield (Vector Laboratories), a glycerol-based medium.
Detection of Neurobiotin and Biotin
Slides were incubated for 15 min in blocking solution (1% gelatin solution from fish skin in PBS). Each slide was then covered with either avidin rhodamine conjugate (10 μg/ml) or streptavidin Alexa 488 conjugate (10 μg/ml) and incubated for 45 min. Then slides were washed twice for 10 min in PBS and mounted in Vectashield.
To control for the degradation of the junction-impermeant tracers in the context of the blastomere cytoplasm, a mixture of 2% dextran-biotin and 2% dextran-fluorescein was injected into one dorsal-animal blastomere of 64-cell stage embryos. The embryos were fixed, sectioned and both the biotin and fluorescein visualized (see Fig. S6, G and H, control panels). Colocalization of the green and the red fluorescence in a single cell demonstrated that both dextran conjugates were stable and that no free biotin or fluorescein was released during the experimental procedures.
Fluorescence Microscopy
Whole embryos and sections were viewed under 4× or 10× objectives on a Nikon Eclipse E800 microscope equipped with Nikon fluorescent filter cubes. Each of the following dyes was visualized by using a specific filter: LY: cube no. 96153 exciter 400–440, barrier 480 nm; rhodamine: cube no. 96157 exciter 528–553, barrier 600–660 nm; fluorescein and Alexa 488: cube no. 96170 exciter 460–500, barrier 510–560 nm; or Alexa 350: cube no. 96100 exciter 330–380, barrier 420 nm.
Image Capture and Processing
Using Image Pro-plus capturing program (Media Cybernetics), images were digitally captured by a Spot camera (Diagnostic instruments, Inc.). Exposure time was adjusted to capture data that most faithfully reflected the visual images. Data were subsequently arrayed figures using Canvas 5 (Deneba Systems).
Synthetic RNA
RNA was transcribed from linearized template Xwnt-8 (Sokol et al. 1991) using the mMESSAGE mMACHINE from Ambion.
2 pg of Xwnt-8 RNA was injected into the marginal zone of two ventral blastomeres of 4-cell stage embryos. 10 pg of Xwnt-8 RNA was injected into the vegetal hemisphere of fertilized eggs.
Online Supplemental Materials
Six additional supplemental figures (Figs. S1–S6) discussed throughout this manuscript are available at http://www.jcb.org/cgi/content/full/150/4/929/DC1.
Figs. S1 and S2.
There is no evidence for junctional transfer of LY in sectioned embryos coinjected with LY and dextran-rhodamine (injection into one dorsal animal blastomere). Fig. S1 shows sections from four different embryos. Fig. S2 shows a whole mount and serial sections of a single embryo.
Fig. S3.
Cytoplasmic bridges allow distribution of dyes at the 16- and 32-cell stage embryos. The colocalization of LY and dextran-rhodamine indicates no gap junctional transfer of LY.
Fig. S4.
Gap junctional transfer of neurobiotin among vegetal blastomeres of normal embryos at the 64-cell stage and at stage 8.
Fig. S5.
UV irradiation does not block gap junctional transfer of neurobiotin.
Fig. S6.
Control panels show lineage tracing of dye injected cells, controls for the detection of neurobiotin and the results of alterations of the dorso-ventral axis of embryos whose siblings were analyzed in dye transfer assays.
Results
LY Transfer in Early Xenopus Embryos
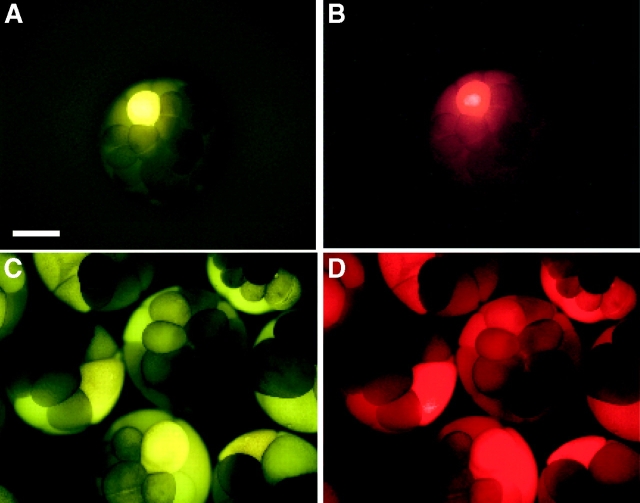
To assess the levels of intercellular communication while controlling for the presence of cytoplasmic bridges, each blastomere was injected with a mixture of intercellular channel-permeant (<1 kD) and -impermeant (>10 kD) molecules. The first set of experiments used LY and dextran-rhodamine (DR), the latter extensively dialyzed to eliminate unbound rhodamine. One animal cell, tier 1 of 32-cell stage embryos, was injected, incubated 10 min then fixed and examined by fluorescence microscopy. As reported in earlier studies, ventral injections produced little evidence of LY transfer (Fig. 1 A), whereas dorsally injected embryos showed variable levels of apparent transfer (Fig. 1 C). However, LY and DR were always coincident (compare Fig. 1A and Fig. B with C and D) suggesting that an extensive network of cytoplasmic bridges, rather than gap junctions, accounted for the movement of the markers. To examine this possibility, serial sections of 36 embryos were analyzed. Fig. 2 compares the whole-mount and sectioned appearance of a single dorsally injected 32-cell stage embryo. Surprisingly, serial sectioning revealed that LY and DR were present in only two cells (Fig. 2, C–H), not in a significant number of cells as suggested by the whole-mount appearance (Fig. 2A and Fig. B). Note that the concentrations of LY and DR are not identical in the sections of each of the cell pairs in Fig. 2, indicating that the cytoplasmic bridge was narrow or transient enough to partially restrict the intercellular transfer of both fluorescent molecules. A limited LY and DR codistribution was consistently observed in all sectioned embryos. Additional comparisons between sectioned and whole-mounted embryos may be seen in Figs. S1 and S2.
Figure 1.
Whole-mount photographs of 16–32-cell stage embryos, coinjected with LY (M r = 457) and dextran-rhodamine (M r = 10,000). Dextran is too large to pass through gap junctional channels and serves as a marker for the injected cell and all cells joined to the injected cell by cytoplasmic bridges. A and B show examples of undetectable dye transfer, while C and D show a sample of embryos where LY appears to spread away from the injected cell. Notice, however, that the nonpermeant dextran shows an identical pattern to the LY. Bar, 0.5 mm.
Figure 2.
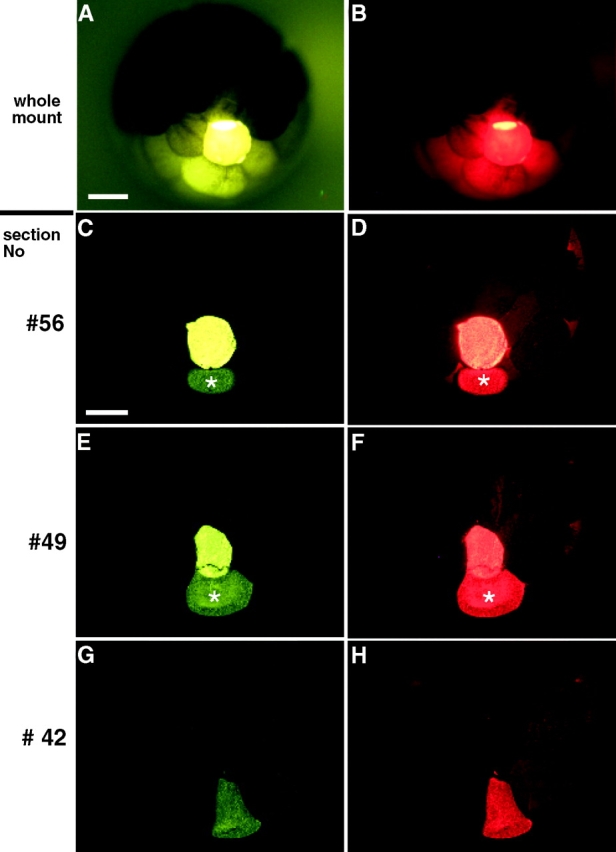
No evidence for junctional transfer of LY was detected in sectioned embryos coinjected with LY and dextran-rhodamine. An embryo in which LY appeared to transfer to adjacent cells (Fig. 2A and Fig. B) was serially sectioned. ∼70 cross sections along the vegetal to animal axis were analyzed. Colocalization of the dyes was always seen, indicating that there was no junctional transfer. (C–F) The dyes were unevenly distributed between the injected cell (very bright green or red cell) and a second cell, which is marked by an asterisk. (G–H) Both dyes remained colocalized in one cell in a plane of section that did not include the injected cell. The presence of dextran-rhodamine in asterisked cells indicates the presence of persistent cytoplasmic bridges with the more brightly stained injected cells. Bars: (A) 250 μm; (C) 200 μm.
Since published reports of LY transfer are based on studies of both 16- and 32-cell stage embryos, we also analyzed 16-cell stage embryos. Guthrie et al. 1988 concluded that during the 16-cell stage, dye freely moved between blastomeres on both the presumptive ventral and dorsal embryonic poles. At the 32-cell stage, 50–70% of dorsal injections and only 35% of ventral injections resulted in LY transfer (Guthrie et al. 1988). To reinvestigate these observations, we collected a population of 16-cell stage embryos (n = 150) and injected a cohort of them (n = 30) every 5 min with the LY/DR mixture. Injections were continued until the embryos were well into the 32-cell stage. None of the serial sections of the embryos in this experiment evidenced LY gap junctional transfer (see Fig. S3).
We have repeated these experiments with two additional negatively charged tracers, Alexa 350 hydrazide (M r = 349.29) and Alexa 488 hydrazide (M r = 570.48). As in the LY experiments, embryos were injected in each case with a mixture of the small tracer together with a large dextran complex, and were studied in serial sections. Both dyes failed to indicate junctional transfer (24 injected embryos with each tracer were analyzed; not shown).
Analysis of dye transfer in whole-mount could produce false positive results due to reflection and scattering of the fluorescent signal. Individual, round blastomeres could act as crude lenses, focusing emitted light into adjacent, yolk-filled cells, which then scatter the light and appear to contain dye. This lens effect could account for the asymmetry in dye transfer between ventral and dorsal blastomeres reported previously (Guthrie 1984; Guthrie et al. 1988; Olson et al. 1991; Olson and Moon 1992; Guger and Gumbiner 1995; Krufka et al. 1998). On the ventral surface, the increased pigment would tend to mask the lens effect, making the ventral blastomeres appear to be less capable of transferring dye.
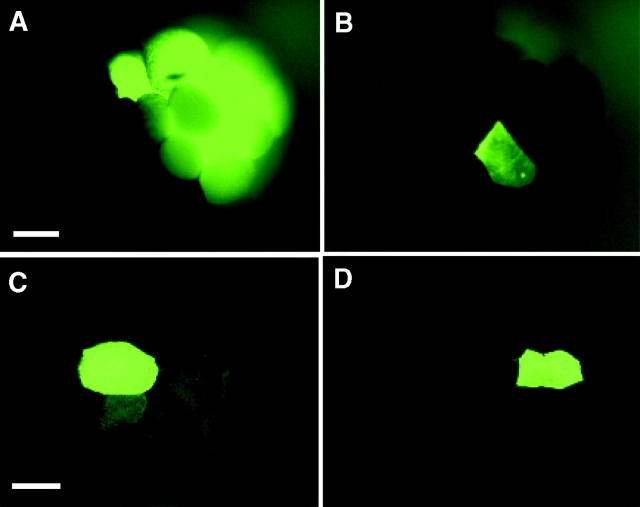
To demonstrate the lens effect, dextran-fluorescein (DF) alone was injected into one dorsal or one ventral blastomere in tier 1 of 32-cell stage embryos (Fig. 3). Whereas DF is too large to pass through gap junctions, an apparent transfer of the tracer to many cells in the dorsally injected embryo can be seen (Fig. 3 A). In the ventrally injected embryo, which was injected with an identical volume of DF, the higher pigment content not only makes the injected cell appear to contain less tracer, but it also shields the lens effect (Fig. 3 B). Sections through these embryos (Fig. 3C and Fig. D) revealed that after either injection, the junction-impermeable DF is confined, as expected, to those cells that are joined by cytoplasmic bridges.
Figure 3.
Intraembryonic reflection and selective masking of fluorescent signal by pigmented cells in whole-mount embryos. 32-cell stage embryos were injected with dextran-fluorescein (DF, mol wt = 10,000) in one dorsal (less pigmented, A) or one ventral (highly pigmented, B) animal cell in tier 1. The lens effect is demonstrated in whole-mount A where fluorescence is seen in many surrounding cells. In contrast, only two less intensely fluorescent cells are seen in whole-mount B. However, sections of embryo A in C and of embryo B in D confirm that after either injection, the junction-impermeable dextran-fluorescein is confined to the injected cell and to those cells that are joined by cytoplasmic bridges. Bars: (A) 250 μm; (C) 200 μM.
Summarizing our LY experiments, the apparent distribution of the junction-permeable marker LY in whole-mounts was very different from that in serial sections. In addition, we were unable to find any evidence for junctional transfer of LY using either approach. Our inability to demonstrate LY transfer was completely inconsistent with published studies (Guthrie 1984; Warner et al. 1984; Guthrie et al. 1988; Nagajski et al. 1989; Olson et al. 1991; Olson and Moon 1992; Guger and Gumbiner 1995; Krufka et al. 1998; Levin and Mercola 1998).
GJIC between Blastomeres in the Xenopus Embryo Revealed by Neurobiotin
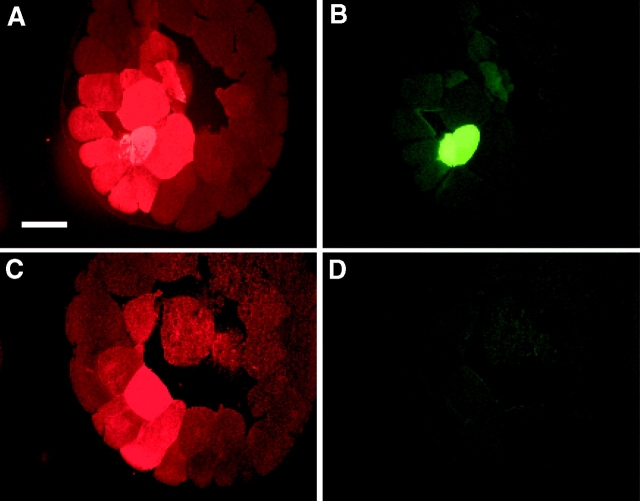
To ascertain if intercellular communication is present in the early embryo, we employed neurobiotin (NB), which can detect intercellular communication in situations where LY does not (Peinado et al. 1993; White et al. 1998). NB was injected in a mixture with either FR or DF and detected in serial sections with either streptavidin–Alexa 488 conjugate or avidin-rhodamine. In the first set of experiments, the neurobiotin mixture was injected into one dorsal-animal tier-1 cell of embryos. NB transfer was evident in 16-cell (Fig. 4A and Fig. B) and 32-cell stage embryos (Fig. 4C and Fig. D). In those examples, both gap junctional transfer (Fig. 4A and Fig. C, cells marked 1 and 2) and passage through a cytoplasmic bridge (Fig. 4C and Fig. D, the cell marked with an asterisk) were detected. As development proceeded, the levels of GJIC increased. This could be clearly seen among animal pole cells in embryos at the 64- (Fig. 4E and Fig. F) and 128-cell stage (Fig. 4, G–K). At the 128-cell stage, junctional transfer from animal pole cells (Fig. 4 G) to vegetal cells (Fig. 4 I) was apparent. In the second set of experiments, neurobiotin mixture was injected into one central-most vegetal blastomere in a second group of embryos and junctional transfer was evident as well (Fig. S4).
Figure 4.
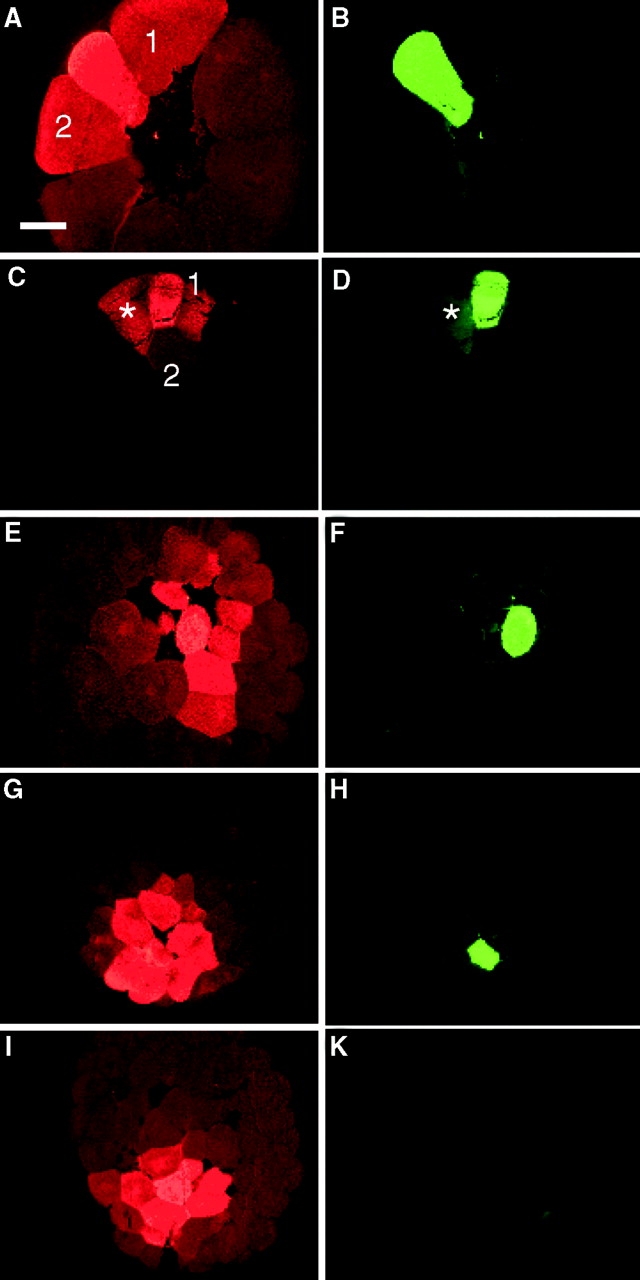
The pattern of neurobiotin (NB) transfer among dorsal blastomeres of normal embryos at the 16-, 32-, 64-, and 128-cell stages. One dorsal blastomere of either 16- (A and B), 32- (C and D), 64- (E and F), and 128- (G–K) cell stage embryos was coinjected with NB and dextran-fluorescein. Sectioned embryos show NB (A, C, E, G, and I) and dextran-fluorescein (B, D, F, H, and K) fluorescence. The 16-cell stage embryo in A, B shows NB transfer into two neighboring animal cells, marked 1 and 2. The injected blastomere of the 32-cell embryo in (C and D) transfers into two animal cells, marked 1 and 2 in C, and is still connected by a cytoplasmic bridge with another animal cell marked with a star in D. The 64-cell stage embryo in E and F shows neurobiotin transfer into at least six neighboring animal cells. The injected blastomere of the 128-cell embryo transfers neurobiotin into >10 animal cells (G and H). Serial sections reveal transfer into more vegetal cells, seen in I, where the injected cell is no longer in the plane of section (K). Bar, 200 μm.
Neurobiotin Does Not Reveal Asymmetry in Gap Junctional Communication
To reinvestigate the possibility of dorso-ventral asymmetry in intercellular communication, we injected NB and DF into a ventral animal cell in tier 1 of 64-cell stage embryos (n = 48) and analyzed serial sections. Dye transfer could be seen in the plane of the injected cell (Fig. 5A and Fig. B), as well as in adjacent sections towards the marginal zone where the injected cell was not seen (Fig. 5C and Fig. D). This pattern of dye transfer was indistinguishable from the data obtained when a single dorsal animal cell was injected in the same batch of embryos (see Fig. 4E and Fig. F). To control for fidelity in the identification of the dorso-ventral embryonic poles, LY was injected into one ventral or one dorsal blastomere of embryos that were analyzed at the tadpole stage (see Fig. S6, E and F, control panels).
Figure 5.
An example of neurobiotin transfer in ventral blastomeres. One ventral blastomere of a normal 64-cell stage embryo was coinjected with neurobiotin and dextran-fluorescein (A–D). The sectioned embryo shows neurobiotin (A and C) and dextran-fluorescein (B and D). More than 10 cells receiving neurobiotin are seen in the animal section (A and B). At least three additional cells that received NB are seen in a different section, vegetal from the injected cell, which is not in this plane of section (D). Bar, 200 μm.
Quantitated data from 12 dorsally injected and 12 ventrally injected embryos are shown in Table . Fluorescent cell profiles were examined on each section for both NB and DF, and only NB-containing cells were scored positively. Thus, the injected cell and all cells joined to the injected cell by cytoplasmic bridges were omitted from the totals. Comparison of the resulting numbers of cells revealed no asymmetry in neurobiotin transfer between dorsal and ventral sides of the embryo. The numbers shown in Table are not the absolute numbers of cells receiving the dye, as all blastomeres were present in multiple sections and were counted more than once. We conclude that GJIC, as demonstrated by neurobiotin transfer, is similar among ventral and dorsal blastomeres.
Table 1.
Equal Neurobiotin Transfer in Dorsally or Ventrally Injected 64-Cell Stage Embryos
| Embryo | Dorsal | Ventral |
|---|---|---|
| 1 | 87 | 66 |
| 2 | 98 | 123 |
| 3 | 59 | 48 |
| 4 | 143 | 100 |
| 5 | 131 | 85 |
| 6 | 72 | 129 |
| 7 | 112 | 107 |
| 8 | 92 | 116 |
| 9 | 103 | 71 |
| 10 | 127 | 52 |
| 11 | 61 | 111 |
| 12 | 120 | 88 |
| Mean | 100.4 | 91.3 |
| SD | 27.5 | 27.4 |
12 dorsally injected and 12 ventrally injected embryos were each analyzed on 70 serial sections each 12 μm thick. Fluorescent cell profiles were counted on each section for both neurobiotin and dextran probes, and only the neurobiotin-positive and dextran-negative cells were scored positively. The results show no significant difference in neurobiotin transfer between dorsally or ventrally injected embryos.
UV-irradiated and Xwnt-8–injected Embryos
It has been reported that intercellular communication was sensitive to manipulations affecting the dorso-ventral fate of the embryo (Nagajski et al. 1989; Olson et al. 1991; Olson and Moon 1992; Guger and Gumbiner 1995; Krufka et al. 1998). To determine if such treatments changed the patterns of NB transfer, we examined embryos ventralized by UV irradiation or dorsalized by exogenous expression of Xwnt-8. Unirradiated and UV-irradiated embryos from the same batch were then injected with a mixture of NB and FD. Analysis of the serial sections from 64-cell stage embryos (n = 48) showed unperturbed dye transfer between dorsal blastomeres in UV-irradiated embryos (Fig. S5). The dose of UV irradiation was adjusted so that 100% of the developing embryos were homogeneously ventralized (Fig. S6, I and J). To examine the effect of dorsalization on patterns of NB transfer, embryos were injected with Xwnt-8 using two separate protocols. In the first, Xwnt-8 RNA was injected under the marginal zone of two ventral blastomeres at the 4-cell stage and then analyzed for GJIC in ventral-animal cells at the 64-cell stage. In the second, Xwnt-8 RNA was injected into fertilized eggs (as described in Olson et al. 1991; Olson and Moon 1992), which were then analyzed for GJIC in ventral-animal cells at the 32-cell stage. In 36 sectioned embryos obtained from these protocols, Xwnt-8 had no effect on the pattern of cell–cell communication as assayed by neurobiotin transfer in ventral blastomeres (data not shown). Also, in 48-sectioned embryos, Xwnt-8 had no effect on LY transfer which was always strictly colocalized with DR as described earlier. In both of the Xwnt-8 protocols, RNA concentrations were adjusted so that 100% of the embryos were homogeneously dorsalized (Fig. S6, I, K, L, and M).
Discussion
Determination of Guidelines for Dye Transfer Experiments
Our attempts to study GJIC in the Xenopus embryo revealed that LY does not transfer between embryonic blastomeres through gap junctions. Furthermore, the analysis of whole-mount embryos using any fluorescent dye is subject to optical artifacts. While whole-mount specimens showed apparent transfer of junction permeant and impermeant molecules, transfer was not observed in paraffin embedded and in frozen (not shown) sectioned specimens. The explanation for this discrepancy is that the intense emission from a single, highly fluorescent cell can be internally reflected within the embryo, illuminating adjacent cells whose light-scattering organelles make them also appear fluorescent. In addition, the asymmetric pigmentation of the Xenopus embryo produces an apparent asymmetry in this light-scattering effect. Thus, inspection of whole-mount embryos is an unreliable method for the assessment of dye transfer between embryonic blastomeres. A rigorous and unambiguous demonstration of gap junctional intercellular communication demands both the coinjection of permeant and impermeant tracers and the examination of sectioned specimens.
Table provides a summary of the studies which used LY transfer to demonstrate asymmetric communication in the Xenopus embryo. None of these studies meet the criteria we have established. Some of these studies did not include injection of impermeant tracers while others injected the impermeant markers independently, in sibling embryos. In all previous studies, quantitative data were collected by whole-mount analysis, although in a few cases, a subset of sibling embryos were sectioned. However, our data show that sibling controls are unreliable because of the transient and asynchronous presence of cytoplasmic bridges. Asynchronous divisions of equivalent blastomeres in a cohort of embryos results in an equivalent lack of synchrony in the completion of cytokinesis and a variable persistence of cytoplasmic bridges. In addition, due to the rapidity of the cell cycle, not only may dye-injected cells cleave at different times than their counterparts in sibling embryos but a cell may also enter a new cycle before it has finished cytokinesis. Therefore, a gap junctional impermeant tracer must always be coinjected with a permeant molecule in each embryo in order to distinguish cytoplasmic bridges from transfer through gap junctions.
Table 2.
Comparison Lucifer Yellow Protocols to Study GJIC in Early Xenopus Embryos
| Study | Measured Lucifer transfer at stages | How passage via cytoplasmic bridges was excluded | All data werecollected from | Control for reflectionand scattered light | |
|---|---|---|---|---|---|
| No. of cells | |||||
| Warner et al. 1984 | 32 | No fluorescent dextran injections Lucifer yellow was injected into one group of embryos and fluorescent dextran was injected into a separate group of embryos | Whole-mounts | Few sibling embryos were analyzed on frozen or plastic sections | |
| Guthrie 1984 | 32 | Whole-mounts | |||
| Guthrie et al. 1988 Olson et al. 1991 | 16–128 32 | Whole-mounts Whole-mounts | |||
| Nagajski et al. 1989 | 32 | No fluorescent dextran injections | Whole-mounts | No sections | |
| Olson and Moon 1992 | 32 | } | Lucifer yellow was injected into one group of embryos and fluorescent dextran was injected into a separate group of embryos | Whole-mounts | No sections |
| Guger and Gumbiner 1995 | 32 | Whole-mounts | No sections | ||
| Krufka et al. 1998 | 32 | Whole-mounts | No sections | ||
| Levin and Mercola 1998 | 8–16 | Fluorescent dextran was injected in a mixture solution with Lucifer yellow | Whole-mounts | No sections | |
| This study | 16–128 | Fluorescent dextran was injected in a mixture solution with Lucifer yellow | Paraffin or frozen sections | All embryos were analyzed on either 12- μm paraffin or 14-μm frozen sections. |
Junctional Communication between Xenopus Blastomeres
In contrast to LY, transfer of NB is evident at all embryonic stages tested. Different rates of LY and NB transfer have been observed in the ocular lens and postnatal neocortex (Peinado et al. 1993; White et al. 1998), likely due to the well-documented dependence of channel permselectivity on connexin composition (Elfgang et al. 1995; Cao et al. 1998). Our data show that gap junctions between Xenopus blastomeres are either impermeable to LY or transfer LY too slowly to be detectable on a time scale compatible with the rapid cell divisions. Our failure to detect LY transfer is subject to technical limitations. Whereas published studies have shown that fixation and embedding do not result in dye redistribution, about half of the injected dye may be lost during these processes (Stewart 1978). If the rate of LY transfer were very low, just on the edge of detection, and if half were lost during processing for sectioning, than such a transfer may not be detectable. Regardless, LY transfer in Xenopus embryos does not occur at the levels and in the asymmetrical patterns previously reported.
Our studies leave open the question of the role of junctional communication in embryonic patterning. NB/fluorescent dextran mixtures clearly revealed communication within and between blastomeres of the animal and vegetal poles. However, communication levels among presumptive dorsal blastomeres were similar to those among ventral blastomeres as visualized by NB. Neither UV irradiation nor exogenous Xwnt-8 expression had any effect on these patterns of cell–cell communication. Thus, our data do not support previously published correlative studies suggesting that communication affects dorso-ventral axis determination in Xenopus. However, we observed significant increases in the levels of communication during early cleavage stages using neurobiotin as a probe and it is possible that other probes would reveal asymmetries that would implicate junctional communication in early patterning events.
Supplemental Material
Acknowledgments
We are grateful for helpful discussions with Drs. A.E. Warner, R.T. Moon, B.M. Gumbiner, J. Heasman, and R.G. Johnson. We are also grateful to Drs. M. Levin and M. Mercola for helpful discussions and sharing of Lucifer yellow reagents.
This work was supported by grant GM18974 (D.A. Goodenough) and GM37751 (D.L. Paul).
Footnotes
The online version of this article contains supplemental material.
Abbreviations used in this paper: DF, dextran-fluorescein; DR, dextran-rhodamine; GJIC, gap junctional intercellular communication; LY, Lucifer yellow.
References
- Cao F.L., Eckert R., Elfgang C., Nitsche J.M., Snyder S.A., Hülser D.F., Willecke K., Nicholson B.J. A quantitative analysis of connexin-specific permeability differences of gap junctions expressed in HeLa transfectants and Xenopus oocytes. J. Cell Sci. 1998;111:31–43. doi: 10.1242/jcs.111.1.31. [DOI] [PubMed] [Google Scholar]
- Cardellini P., Rasotto M.B., Tertoolen L.G., Durston A.J. Intercellular communication in the eight-cell stage of Xenopus laevis developmenta study using dye coupling. Dev. Biol. 1988;129:265–269. doi: 10.1016/0012-1606(88)90180-7. [DOI] [PubMed] [Google Scholar]
- Elfgang C., Eckert R., Lichtenberg-Fraté H., Butterweck A., Traub O., Klein R.A., Hülser D.F., Willecke K. Specific permeability and selective formation of gap junction channels in connexin-transfected HeLa cells. J. Cell Biol. 1995;129:805–817. doi: 10.1083/jcb.129.3.805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guger K.A., Gumbiner B.M. β-Catenin has wnt-like activity and mimics the Nieuwkoop signaling center in Xenopus dorsal-ventral patterning. Dev. Biol. 1995;172:115–125. doi: 10.1006/dbio.1995.0009. [DOI] [PubMed] [Google Scholar]
- Guthrie S. Patterns of junctional communication in the early amphibian embryo. Nature. 1984;311:149–151. doi: 10.1038/311149a0. [DOI] [PubMed] [Google Scholar]
- Guthrie S.C., Turin L., Warner A.E. Patterns of junctional communication during development of the early amphibian embryo. Development. 1988;103:769–783. doi: 10.1242/dev.103.4.769. [DOI] [PubMed] [Google Scholar]
- Krufka A., Johnson R.G., Wylie C.C., Heasman J. Evidence that dorsal-ventral differences in gap junctional communication in the early Xenopus embryo are generated by beta-catenin independent of cell adhesion effects. Dev. Biol. 1998;200:92–102. doi: 10.1006/dbio.1998.8951. [DOI] [PubMed] [Google Scholar]
- Levin M., Mercola M. Gap junctions are involved in the early generation of left-right asymmetry. Dev. Biol. 1998;203:90–105. doi: 10.1006/dbio.1998.9024. [DOI] [PubMed] [Google Scholar]
- Nagajski D.J., Guthrie S.C., Ford C.C., Warner A.E. The correlation between patterns of dye transfer through gap junctions and future developmental fate in Xenopusthe consequences of UV-irradiation and lithium treatment. Development. 1989;105:747–752. [Google Scholar]
- Olson D.J., Christian J.L., Moon R.T. Effect of wnt-1 and related proteins on gap junctional communication in Xenopus embryos. Science. 1991;252:1173–1176. doi: 10.1126/science.252.5009.1173. [DOI] [PubMed] [Google Scholar]
- Olson D.J., Moon R.T. Distinct effects of ectopic expression of Wnt-1, Activin B, and bFGF on gap junctional permeability in 32-cell Xenopus embryos. Dev. Biol. 1992;151:204–212. doi: 10.1016/0012-1606(92)90227-8. [DOI] [PubMed] [Google Scholar]
- Peinado A., Yuste R., Katz L.C. Extensive dye coupling between rat neocortical neurons during the period of circuit formation. Neuron. 1993;10:103–114. doi: 10.1016/0896-6273(93)90246-n. [DOI] [PubMed] [Google Scholar]
- Peng H.B. Solutions and protocols. Methods Cell Biol. 1991;36:657–662. [PubMed] [Google Scholar]
- Sokol S., Christian J.L., Moon R.T., Melton D.A. Injected Wnt RNA induces a complete body axis in Xenopus embryos. Cell. 1991;67:741–752. doi: 10.1016/0092-8674(91)90069-b. [DOI] [PubMed] [Google Scholar]
- Stewart W.W. Functional connections between cells as revealed by dye-coupling with a highly fluorescent naphthalimide tracer. Cell. 1978;14:741–759. doi: 10.1016/0092-8674(78)90256-8. [DOI] [PubMed] [Google Scholar]
- Warner A.E., Guthrie S.C., Gilula N.B. Antibodies to gap-junctional protein selectively disrupt junctional communication in the early amphibian embryo. Nature. 1984;311:127–131. doi: 10.1038/311127a0. [DOI] [PubMed] [Google Scholar]
- White T.W., Goodenough D.A., Paul D.L. Targeted ablation of connexin50 in mice results in microphthalmia and zonular pulverulent cataracts. J. Cell Biol. 1998;143:815–825. doi: 10.1083/jcb.143.3.815. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.