Abstract
Functional imaging experiments, in particular positron-emission tomography and functional magnetic resonance imaging, can be analyzed either in psychological terms or on the basis of neuroscience. In the usual psychological interpretation, stimulations are designed to activate specific mental processes identified by cognitive psychology, which are then localized by the signals in functional imaging experiments. An alternate approach would be to analyze experiments in terms of the neurobiological processes responsible for the signals. Recent in vivo 13C NMR measurements of the glutamate-to-glutamine neurotransmitter cycling in rat and human brains facilitate a neuroscientific interpretation of functional imaging data in terms of neurobiological processes since incremental neurotransmitter flux showed a 1:1 stoichiometry with the incremental rate of glucose oxidation. Because functional imaging signals depend on brain energy consumption, a quantitative relationship can be established between the signal (S) and the specific neurochemical cerebral neurotransmitter activity (N) of glutamate-to-glutamine neurotransmitter cycling. The quantitation of neuronal activity proposed has implications for the psychological design and interpretation of functional imaging experiments. Measurements of the neurotransmitter cycling flux at rest in functional imaging experiments suggest that performing cognitive tasks and sensory stimulations increases neurotransmitter cycling by only 10–20%. Therefore it cannot be assumed that reference state activities are negligible, nor that they are constant during stimulation.
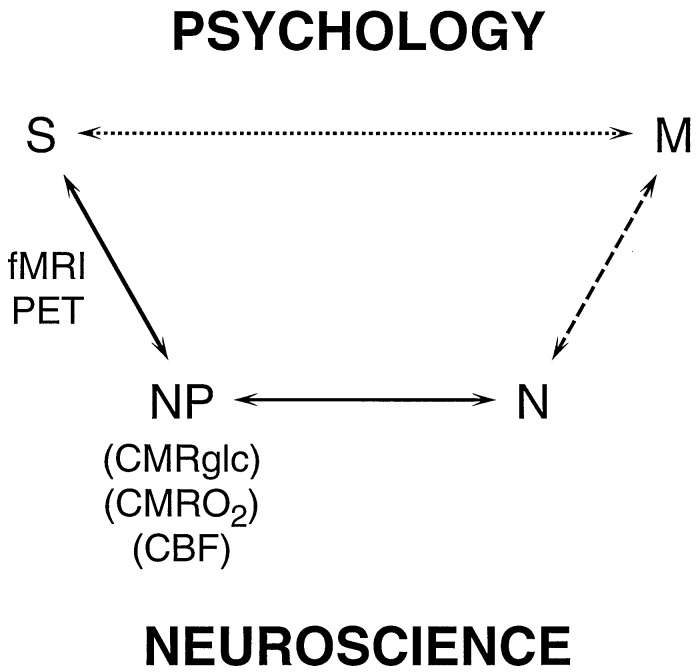
In this period of intense research in the neurosciences, nothing is more promising than functional magnetic resonance imaging (fMRI) and positron emission tomography (PET) methods, which localize brain activities. These functional imaging methodologies map neurophysiological responses to cognitive, emotional, or sensory stimulations (1–4). The rapid experimental progress made by using these methods has encouraged widespread optimism about our ability to understand the activities of the mind on a biological basis. However, the relationship between the signal and neurobiological processes related to function is poorly understood, because the functional imaging signal is not a direct measure of neuronal processes related to information transfer, such as action potentials and neurotransmitter release. Rather, the intensity of the imaging signal is related to neurophysiological parameters of energy consumption and blood flow. To relate the imaging signal to specific neuronal processes, two relationships must be established, which are schematicized in the lower pathway of Fig. 1. The first relationship is between the intensity of the imaging signal (S) and the rate of neurophysiological energy processes (NP), such as the cerebral metabolic rates of glucose (CMRglc) and of oxygen (CMRO2). The second and previously unavailable relationship is between the neurophysiological processes (NP) and the activity of neuronal processes (N). It is necessary to understand these relationships to directly relate functional imaging studies to neurobiological research that seeks the relationship between the regional activity of specific neuronal processes (N) and mental processes (M).
Figure 1.
Schematic relations between the signal (S) obtained in functional imaging experiments and mental processes (M). In the usual experimental plan and interpretation, based on psychology, a direct relationship between S and M is assumed, as represented by the upper pathway. The definition of M is based on psychology, while the imaging experiment serves to localize and quantitate the brain activity identified with the process. The lower pathway, Neuroscience, assumes that M has a molecular and cellular basis, which is broken into three steps leading to S. The signal, S, in fMRI or PET experiments, is primarily a measure of the neurophysiological parameters (NP) of cerebral metabolic rate of glucose consumption (CMRglc), cerebral metabolic rate of oxygen consumption (CMRO2) or CBF. PET methods have been developed for measuring each of these three parameters separately, while fMRI signals respond to differences in the changes of CBF and CMRO2, whose quantitative relationships are being investigated. CMRO2 and CMRglc measure cerebral energy consumption, while ΔCMRO2 and ΔCMRglc measure its increment. The relation between (NP) the neurophysiological measure of energy consumption and neuronal activity (N) has been clarified by the 13C MRS experiments (8–10). These recent findings allow measurements of S to be converted into measures of N, which places us squarely facing the unsolved “hard” problem of neuroscience, i.e., what is the relationship between M and N?
The standard interpretation of functional imaging in the study of mental processes does not incorporate the neurobiological basis of the signal, as shown by the lower pathway in Fig. 1. Instead, an approach based on cognitive psychology is used to interpret the signal, as shown by the upper pathway in Fig. 1 (6, 7). An increase in signal intensity above resting values is interpreted as directly measuring the activity of a mental process in a specific brain region. Mental processes are defined by using a psychological analysis of the brain response to sensory, behavioral, or cognitive tasks. The neurobiological processes responsible for the signal are not included in the interpretation but are assumed to provide a reliable connection between the signal and mental processes. The potential importance of strengthening these assumptions by incorporating neurobiological information into the interpretation of functional imaging experiments has been recognized. In a recent review (5) of functional imaging, Raichle concluded, “We have at hand tools with the potential to provide unparalleled insights into some of the most important scientific, medical, and social questions facing mankind. Understanding those tools is clearly a high priority.”
In this paper, we address the relationship between the functional imaging signal and functional neuronal processes, using a recently proposed model based on in vivo 13C NMR (8–10) and isolated cell experiments (11) of the molecular and cellular coupling between neurotransmitter release and cerebral neuroenergetics. This model quantitatively relates CMRO2 and CMRglc in cortical regions to the rate of glutamate/γ-aminobutyric acid neurotransmitter release and reuptake by the astrocytes. The ability to interpret imaging measurements of CMRO2 and CMRglc in terms of neurotransmitter flux provides a measurement of brain functional activity in both the presence and absence of external stimuli. The high level of activity observed in the “resting” brain does not support the implicit assumption, generally used to interpret functional imaging, that brain activity is negligible at rest.
The Neuroenergetic Basis of the Functional Imaging Signal.
Under normal physiological conditions, glucose metabolism provides almost all of the energy for the brain (12). Under steady-state conditions, 90–95% of the glucose consumed by the brain is oxidized to CO2, leading to the production of ≈34 ATP molecules per glucose molecule. During transitions to higher levels of activity in sensory systems, CMRglc increases by a slightly greater percentage than does CMRO2 (13, 14). However, because of the production of only 2 ATP molecules per glucose molecule in glycolysis, most of the increase in energy production still occurs because of oxidative glycolysis. If these transitional couplings are appropriately accounted for, regional brain energy consumption may be determined from measurements of either the oxidative component of CMRglc or CMRO2.
Imaging signals obtained in different PET experiments measure the regional rate of specific neurophysiological processes (NP) of CMRglc (15), CMRO2 (16), and cerebral blood flow (CBF) (17). The fMRI signal is an indirect measure of the difference in changes of CMRO2 and CBF during a stimulation (18–20). For fMRI, methodological issues remain as to how accurately the imaging signal may be deconvoluted to measure the separate rates of ΔCBF and ΔCMRO2. We assume that these methodological issues are resolvable and treat the imaging signals as being able, in principle, to provide a measure of ΔCMRO2 or ΔCMRglc and as being able to provide, in some PET experiments, measurements of their absolute values. Symbolically, we assume that the imaging experiments can be interpreted to give
 |
1 |
Functional neuronal processes (N) are mediated by chemical reactions that require ATP. Therefore, any measure of total energy consumption by the neurophysiological parameters will include the energy required for all these chemical reactions. The neuronal processes of interest in functional imaging are determined by short-term information transfer between neurons and include action potentials, neurotransmitter release and reuptake, and excitatory and inhibitory post- and pre-synaptic potentials. However, processes not associated with short-term information transfer also require energy. These processes are sometimes referred to as housekeeping functions (H), which include protein and membrane synthesis and turnover and maintenance of resting ionic gradients.
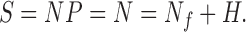 |
2 |
Symbolically, after the signal S is expressed in units of energy consumption (parameters of NP), it is a sum of the energetics of functional neuronal processes (Nf) plus housekeeping processes (H).
Examination of Eq. 2 shows that, because of the requirement for energy by all brain processes, the imaging signal cannot be used to distinguish functional Nf from total neuroenergetics (N). As discussed below, the majority of functional imaging studies attempt to circumvent this limitation by assuming that the change in signal (and therefore energetics) during performance of a task or during sensory stimulation is only because of functional processes. Interpretation is then based largely on the regional difference in signals between states. While this assumption may be valid, the standard interpretation is complicated by the fact that we do not know the fraction of total signal dedicated to functional processes. An additional complication is that, since all functional neuronal processes require energy, the degree to which any specific process is altered cannot be determined from the change in signal intensity unless the energetic consumption of that specific process is known.
A Model Coupling Glutamate/GABA Neurotransmitter Release to Functional Neuroenergetics.
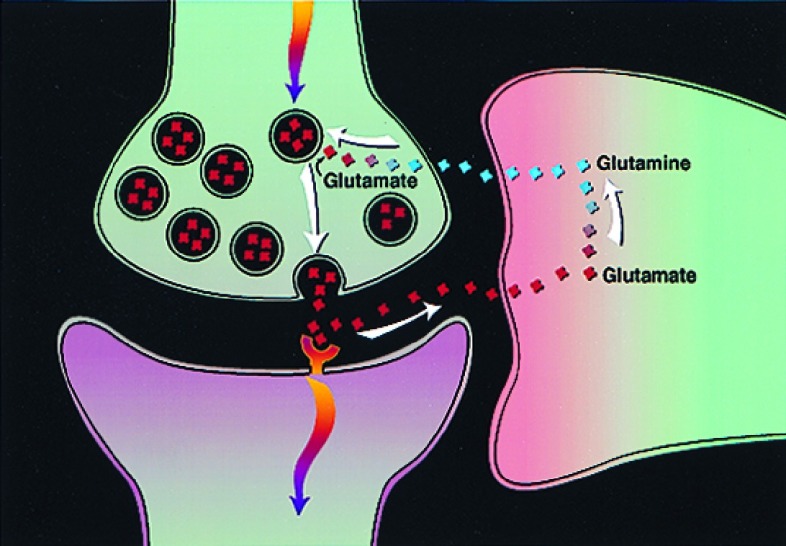
Evaluation of contributions from specific neurochemical processes to Nf is needed to relate the functional imaging signal (S) to neuronal processes on the molecular level. One such process is astrocytic uptake of synaptically released glutamate and GABA and their concomitant recycling to the neurons. Glutamate and GABA are the major excitatory and inhibitory neurotransmitters, respectively, in human cortex. Combined, they account for approximately 90% of cortical synapses (21). Information transfer between cortical neurons is largely mediated, therefore, by the release of GABA and glutamate. A large fraction of released glutamate and GABA is taken up by astrocytic end processes that surround cortical synapses (22, 23), as indicated in Fig. 2. In astrocytes, the glutamate or GABA may be inactivated by glutamine synthetase-catalyzed conversion to glutamine (24). Glutamine may then be released by the astrocyte and, subsequently, through the action of phosphate-activated glutaminase and glutamate dehydrogenase, may be used to replenish neurotransmitter pools of glutamate and GABA. In this section, we describe how the rate of this glutamate/GABA/glutamine (Glu/GABA/Gln) cycling, Vcycle, has been measured by in vivo magnetic resonance spectroscopy (MRS) (8–10). From these measurements, combined with cellular biochemistry studies by Magistretti and coworkers (11), we have developed a model in which cortical functional neuroenergetics (Nf) are directly coupled to Vcycle.
Figure 2.
Proposed pathway of glutamate/glutamine neurotransmitter cycling between neurons and glia (22, 23), whose flux has been quantitated recently by 13C MRS experiments (9). Action potentials reaching the presynaptic neuron cause release of vesicular glutamate into the synaptic cleft, where it is recognized by glutamate receptors post-synaptically and is cleared by Na+ -coupled transport into glia. There it is converted enzymatically to glutamine, which passively diffuses back to the neuron and, after reconversion to glutamate, is repackaged into vesicles. The rate of the glutamate-to-glutamine step in this cycle (Vcycle), has been derived from recent 13C experiments (8–10).
(i) In vivo 13C MRS Measurements of the Rate of the Glu/GABA/Gln Vcycle.
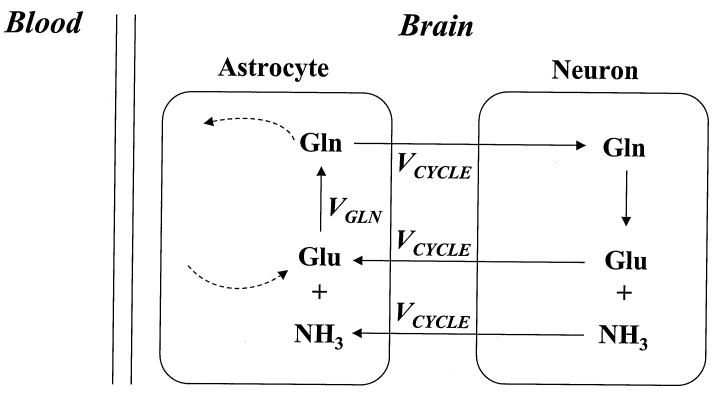
The Glu/GABA/Gln cycle (Fig. 2) was first proposed in the 1970s, on the basis of 14C isotopic studies (see ref. 23 for review). Subsequent evidence for the pathway was obtained by the cellular localization of intermediates [glutamine, mainly in glia (24), and glutamate in neurons (25)], and of the enzymes glutamine synthetase in glia (26) and phosphate-activated glutaminase in neurons (24). Before the in vivo MRS results described below, the in vivo rate of this cycle was believed to be a small fraction of the rate of glucose metabolism, on the basis of the finding of low rates of 13C label incorporation into glutamine from glucose in brain slice preparations (27, 28). In vivo MRS is similar to MRI, except that it uses the slight differences in resonance frequency between different chemical groups to measure the regional concentrations of brain chemicals, including glutamate, GABA, and glutamine. In combination with infusions of 1-13C-labeled glucose, the rates of label incorporation into these amino acids were measured and modeled to obtain the rate of the tricarboxylic acid cycle, which is determined from label incorporation into the glutamate pool (29–31) and the rate of glutamine synthesis (32, 33), determined by the rate of label appearance in the glutamine pool. The rate of the tricarboxylic acid cycle measured by MRS, from the label incorporation into the glutamate pool, can be converted into values of oxidative glucose consumption [CMRglc(ox)], which are in good agreement with traditional measures of oxidative glucose consumption in both anesthetized rats (31) and awake humans (30, 32, 33). These 13C MRS studies in rat and human cortex found that, in contrast with results in brain slices, the labeling from infused 13C-enriched glucose to glutamine was a significant fraction of total 13C label incorporation in both rat (8) and human (32, 33) cortex. To determine the fraction of glutamine labeling resulting from neurotransmitter cycling (i.e., to distinguish Vcycle from the experimental value of VGln, which is the measured total flux from glutamate to glutamine), we reevaluated the contributions of other pathways of glutamine labeling. The most significant alternate pathway is the de novo synthesis of glutamine to remove ammonia from the brain. For this reevaluation, we extended the standard formulation of the cycle to include the contributions of ammonia disposal to VGln (Fig. 3). A comparison of 13C- to 15N-labeling of glutamine at different levels of plasma ammonia showed that ≈90% of glutamine synthesis comes from the neurotransmitter cycle under conditions of normal plasma ammonia (i.e., Vcycle ≈ 0.9 VGln). Therefore, a measurement of glutamine labeling using 13C NMR from 1-13C glucose provides a direct measure of the rate of Vcycle, after a small correction.
Figure 3.
The experimental measure of VGln, the flux from glutamate to glutamine, includes, in addition to Vcycle, contributions from other pathways, as indicated by dashed lines, which have been shown to require small (≈10%) corrections to VGln to obtain Vcyle.
(ii) Model Relating the Glu/GABA/Gln Cycle to Functional Neuroenergetics.
The measurement of the Vcycle by 13C MRS has the advantage of high specificity but is limited at present by low spatial resolution relative to PET and MRI functional imaging methods. In this section, we present experimental and theoretical evidence that a large fraction of the neuroenergetics is directly coupled to glutamate and GABA neurotransmitter release. Consequently, the functional imaging determination of the neuroenergetic parameters, CMRglc or CMRO2, can be related to neurotransmitter cycling, Vcycle.
To study the relationship between the rate of the Glu/GABA/Gln cycle and neuroenergetics, 13C NMR was used to simultaneously measure Vcycle and CMRglc(ox) at different levels of anesthesia ranging from isoelectric to mild anesthesia (9, 10). The results, shown in Fig. 4, indicate that, above isoelectricity, there is a linear relationship between Vcycle and CMRglc(ox), with a close to 1:1 stoichiometry. An extrapolation to the awake resting state, where CMRglc(ox) is known to be ≈0.8 μmol/min per mg, shows that Vcycle is ≈85% of the CMRglc(ox). The linear relationship between Vcycle and CMRglc(ox), above a low baseline value of CMR, suggests that changes in Vcycle may be directly determined from experimental changes in CMRglc(ox).
Figure 4.
Experimental results of Vcycle and the rates of glucose oxidation measured simultaneously in each rat going from N2O/morphine to α-chloralose to pentobarbital (least active), at graded anesthesia. The best fit line gives CMRglc(ox) = 1.04 Vcycle + 0.10. The slope shows that each mole of neurotransmitter glutamate cycling requires the oxidation of one mole of glucose. The awake, resting state has CMRglc(ox) ≈0.8 μmol/min/g, which means that under this condition ≈85% of the brain energy consumption is dedicated to Vcycle (9).
A chemical interpretation of the relationship between Vcycle and CMRglc(ox) is provided by Magistretti and Pellerin’s studies of glial cell suspensions (11). They found that glucose consumption in cultured glia increased in proportion to extracellular glutamate concentration in the range expected in the synaptic cleft. The incrementally consumed glucose was not oxidized but was transported into the medium as lactate. They proposed a model in which glucose consumption in the astrocyte may be coupled to functional neuronal energy requirements through the use of glycolytic ATP for transporting neurotransmitter glutamate into the astrocytic end process. For each glutamate molecule transported into the astrocyte, 3 Na+ molecules are cotransported, which the Na+/K+ ATPase must pump out to maintain a steady state. If this ATP is produced by nonoxidative glycolysis, one-half of a glucose molecule is consumed per glutamate molecule transported into the astrocyte. The lactate produced then mainly diffuses to the neurons, where it is oxidized to supply the neurons’ functional energy requirements.
The 13C MRS results imply that, if this mechanism is operational, it can account for essentially all of the glucose metabolism being used to provide energy for functional neuronal processes. To extend this model so that it would describe the Glu/GABA/Gln neurotransmitter cycle in vivo, one more ATP is required per glutamate for the glutamine synthetase reaction. The astrocytic stoichiometry between glucose conversion to lactate and glutamate uptake would then be 1:1, which agrees with that measured in the 13C MRS experiment (see Fig. 4). A similar stoichiometry would be expected for GABA, which is also cotransported with Na+ cations.
These results show that changes in neuroenergetics with functional activity are, within experimental error, equal to changes in neurotransmitter cycling [ΔCMRglc(ox) = ΔVcycle]. Therefore, to a first order, when functional images are quantitated in terms of changing neuroenergetics (ΔCMRglc or ΔCMRO2), they may be considered to map incremental neurotransmitter release. At present, several points in this model need to be additionally established. Most important are the need for accurate VGln measurements in the awake and stimulated human, the need for more in vivo mechanistic evidence for the cycle, and the mechanistic linkage to glucose metabolism proposed by Magistretti and coworkers. Although the model may require modification and additions, we believe that the present results provide the basic interpretation of functional imaging studies, in which glutamate-to-glutamine neurotransmitter cycling rates can be determined from the neurophysiological measurements of cerebral energetics.
Evaluation of the Neuroenergetic Basis of Functional Imaging Studies.
In general, the paradigms used to design and interpret functional imaging studies have avoided the neurobiological basis of the signals (6). Instead, they are designed to induce a mental activity (M) from psychologically constructed tasks and to use the signal S to locate and quantitate the mental activity so defined. However, as described, the signal arises from the neuroenergetic requirements of the sum of the biochemical processes in an imaging voxel. Therefore, implicit in the psychological approach are assumptions about the relationship between neuroenergetics and mental processes. In this section, we describe a generalized functional imaging paradigm explicitly in terms of the neuroenergetic processes underlying the signal. It is shown that the standard interpretation based on psychology depends on assumptions about the fraction of the resting and task-induced neuroenergetics that derive from functional neuronal processes. By using the relationship that we have established between neuroenergetics and neurotransmitter cycling, these assumptions are reexpressed as assumptions about neurotransmitter cycling and are evaluated by using recent PET and MRS results.
In the majority of functional imaging studies, sensory processes, as well as complex mental processes such as reading, speech, or learning, are considered to be separable into discrete mental components (6). It is hypothesized that there are specialized computational regions localized within the brain, defined as modules that perform these component processes. Each module is treated, to a first approximation, as an input–output computational device. In a typical experiment, two tasks are designed that are assumed to differ only in the activity of a specified mental function. The difference in the functional imaging signal (Sit) between the tasks (t) is then used to identify experimentally the location and magnitude of the modular activity (Mt). To simplify the following description, one of the task states is chosen as the baseline state in which the subject neither is stimulated nor is asked to perform mental processing (although these results are equally applicable to the differences between two tasks). The signals obtained in a specific brain region in the task and baseline states are then described in terms of neuroenergetics as:
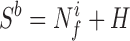 |
3 |
and
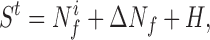 |
4 |
and where ΔNf is the change in the energetics of functional neuronal processes required for the region to perform the task, Nfi is the energy required for internally generated neuronal processes, and H is the energy required for housekeeping functions. The baseline functional neuronal processes are all internally generated, as opposed to externally induced, activity associated with task performance. If the difference in signal reflects, as assumed, only the requirements of the stimulated component of mental activity, as a consequence, maintenance functions H and Nfi must remain constant. In this case, the difference signal yields
 |
5 |
as shown in Fig. 5. On the basis of the psychological assumption that tasks may be designed that specifically activate designated mental processes, the increase in neuroenergetics represented by the increase in signal may be assigned exclusively to the hypothesized module:
 |
6 |
Evaluating the validity of a modular model of brain function ultimately depends on understanding the relationship between the activity of neuronal processes and the mental processes they support. At present, this relationship, which is assumed generally to be based on theories of cognition, is not able to provide a definitive answer (34); one can only test this assumption vs. other information (35). However, examination of Eqs. 3–6 indicates that the ability to associate changes in the imaging signal with specific mental modules depends on two additional assumptions about the neuroenergetic basis of the signal, which we propose are now partially addressable experimentally.
Figure 5.

Schematic representation of the signals obtained in functional imaging differencing experiments. PET measurements of CMRglc and CMRO2 directly measure S and (S + ΔS) under both conditions, although usually only the difference ΔS is used to localize and quantitate the task activation. On the other hand, fMRI signals include strong contributions in both conditions from pure imaging signals, so that in these experiments only ΔS is readily related to task activations.
(i) The incremental functional neuroenergetics of the region during the task are sufficient and necessary to support the discrete mental process or module. This assumption is necessary to separate the energetics required for internal and task-induced functional neuronal processes.
(ii) The neuroenergetics required for internally generated functional processes (Nfi and housekeeping functions (H) do not change during stimulation. This assumption can be adapted readily to the differencing between two tasks, but for simplicity we have stayed with a baseline comparison.
Since all functional neuronal processes present in the task state are also present in the baseline state, these assumptions of being able to isolate task-induced neuronal processes on the basis of changes in the signal are met trivially if the internally general functional neuroenergetics are small with respect to the changes, so that any changes in Nfi would be negligible. Symbolically:
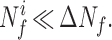 |
7 |
The expression of the functional imaging signal in terms of neurotransmitter cycling allows a neurobiological assessment of the limiting case expressed in inequality 7. On the basis of the model wherein the glucose requirements for functional processes are coupled in a close to 1:1 stoichiometry with the Glu/GABA/Gln neurotransmitter cycle, inequality 7 may be reexpressed as:
 |
8 |
If inequality 8 were satisfied, the assumptions about the neuroenergetic basis of the differencing method would be strongly supported. A comparison of the fractional increase in signal intensity for representative cognitive and sensory studies in humans, in which ΔCMRO2 was imaged, is shown in Table 1. On the basis of animal studies, it is known that approximately 85% of the baseline energy consumption is coupled to Glu/GABA/Gln neurotransmitter cycling in a 1:1 stoichiometry (9). Assuming the same stoichiometry for the increment, one may predict a ΔVcycle fi, which is at maximum only 30% of the baseline rate. Even for a more conservative figure of 50%, based on the decrease in whole-brain CMRO2 during isoelectricity in humans (which will underestimate the cortical decreases) (14), Vcycle fi is still a large fraction of the total Vcycle during a task. The calculation of neurotransmitter cycling from cortical energy consumption in humans is supported by recent 13C MRS studies that have measured rapid glutamine labeling in resting human cortex (32, 33). Consequently, there is no support, on the basis of a theoretical quantitation of neurotransmitter cycling, for isolating the activity of the neuronal processes induced by the task from the increase in the imaging signal. Even in the absence of a task, there is substantial neurotransmitter cycling and associated neuroenergetic requirements. This result does not disprove the standard interpretation, which would still be valid if Nfi and H, instead of being negligible, were in fact constant when the task was changed. However, because Nfi is large, the possibility of a change in the contribution of Nfi to the measured change in signal must be considered.
Table 1.
Experimental energy changes upon stimulation
| Stimulation | ΔCMRglc, % | ΔCMRO2, % | Ref. |
|---|---|---|---|
| Visual | 51 | 5 | Fox et al. (13) |
| 28 | 28 | Marrett et al. (41) | |
| 29 | 29 | Marrett et al. (41) | |
| 16 | Davis et al. (42) | ||
| 23 | Chen et al. (43) | ||
| 24 | Reivich et al. (44) | ||
| Average | 31 | 20 | |
| Cognitive | 12 | Roland et al. (45) | |
| Seizure | 400 | 267 | Borgstrom et al. (46) |
Partially compiled in Reference 14.
Implications for Psychological Models of Functional Imaging.
An alternate psychological model would be to associate not only the increments but the entire functional neuroenergetics in a region with mental processes. In this interpretation, there is no distinction between internally and externally generated neuronal activity, i.e., Nfi and Nft. All functional neuronal processes within a region contribute to the supported mental processes, and
 |
9 |
Such a viewpoint has been expressed by Changeux (36), by comparing the neurobiological correlates of imagined and actual images. On the basis of the high amount of baseline neuronal electrical activity, he proposed that models that include internal mental processes should be evaluated experimentally. Our assignment of high neurotransmitter activity at rest agrees with his notice of high electrical activity.
Since the psychological basis of brain activity is at present still a hypothesis, it is noteworthy that many find an inclusive view of the brain more appealing than the model from cognitive psychology. The philosopher John Searle has led the attack by saying, “In the philosophy of mind, obvious facts about the mental such as that we all really do have subjective conscious mental states . . . are routinely denied by many, perhaps most, of the advanced thinkers in the subject” (37).
These alternative views of subjective contributions to mental activity are not directly required by our findings. However, including the subjective, this position would accept that internally generated neuronal activity plays a role in mental processes and should not be neglected, which is in accord with our findings of high neurotransmitter activity in the resting state.
In addition to allowing this alternative view of the brain, which could generate different imaging experiments, we provide examples below to illustrate how the inclusion of internally generated neuronal activity in mental processes facilitates the psychological interpretation of present functional imaging experiments.
(i) The relationship between the signal and the activity of a mental process.
In an ideal psychological model of functional imaging, it would be possible to determine the relative activity of a mental process from the signal intensity. The high activity of neuronal processes in the resting state significantly impacts this quantitation. For example, consider a comparison of the brain signals between two tasks that activate the same mental process. Suppose that in the first task the signal increases above baseline by 1% and in the second task it increases by 2%. An interpretation on the basis of the incremental signal would be that the second task uses twice the modular activity as the first. However, if the whole rather than the incremental neuronal activity is related to the mental process, the difference in activity between the tasks is only about 1%. In actuality, it is unlikely that the activity in any region may be assigned to a single mental process. However, this example illustrates the significance of evaluating the contributions of the total neuronal activity to brain function.
(ii) The Presence of Negative Signals During Task.
Recent studies have found a decrease in the imaging signal in some brain regions during a task relative to the baseline control state (38). These decreases have been interpreted, in the standard paradigm, as paradoxical (5), since it is implicitly assumed that if there are mental processes occurring at rest, they are distinct from those induced by the task and they would be stable. However, a decrease of signal in certain tasks would not be unexpected if the same mental processes were occurring in the baseline state. The activity of these processes could simply be lower in the task state. An analogous interpretation would explain the observation of regions that do not change in activity during tasks but are known from lesion and electrophysiological studies to be important for mental processing (38). In the incremental view, the absence of statistically significant increments would not allow activity in those regions.
Neurobiology.
An alternative to designing functional imaging studies to test psychological theories is to design studies to test theories at the neurobiological level. By quantitating the functional imaging signal in terms of neuronal processes, such studies are possible. Although details of the model remain to be confirmed, corrected, or evaluated experimentally, it is clear that a framework now exists for converting the signals S in a functional imaging experiment into the absolute and incremental values of Glu/GABA/Gln release and cycling. The steps from S → Nf can be quantified so that it is possible to face the “hard problem” of neurobiology, i.e., Nf → M, more directly in the near future. For example, theories about local information processing in a neuronal network could be tested on the basis of their predictions about the effects on the total neurotransmitter release in a region. The quantitation proposed here is not the only quantitation possible. For example, the development of quantitative electrical mapping would provide information on the pattern of neuronal activity. Recent developments in MRS provide the promise of further definition of the neurotransmitter cycling flux into excitatory and inhibitory fractions (39).
The integration of the neurotransmitter model into the neuroenergetics explored by functional imaging offers an opportunity to advance neuroscientific understanding. This integration combines the high spatial resolution of functional imaging methods with the definite neurochemical interpretations of the signals. In addition to providing a stepping-stone toward an understanding of mental activities, this interpretation also allows for correlations to be sought between cellular and molecular neuroscience studies. Finally, we repeat the credo of neuroscientists expressed by Francis Crick, “The scientific belief is that our minds—the behavior of our brains —can be explained by the interactions of nerve cells (and other cells) and the molecules associated with them” (40). It is our hope that the neurotransmitter interpretation of functional imaging can serve this goal.
Acknowledgments
The authors acknowledge helpful comments by Profs. Elizabeth Phelps and Bruce Wexler and grant support by the National Institutes of Health (R01 DK27121).
ABBREVIATIONS
- PET
positron emission tomography
- fMRI
functional magnetic resonance imaging
- CMRglc
cerebral metabolic rate of glucose
- CMRO2
cerebral metabolic rate of oxygen
- CBF
cerebral blood flow
- CMRglc(ox)
oxidative glucose consumption
- GABA
γ-aminobutyric acid
- Glu/GABA/Gln
glutamate/GABA/glutamine
- MRS
magnetic resonance spectroscopy
References
- 1. Raichle M E. In: Higher Functions of the Brain, Handbook of Physiology: The Nervous System. Plum F, Montcastle V, editors. Vol. 5. Washington, DC: Am. Physiol. Soc.; 1987. pp. 643–674. [Google Scholar]
- 2.Sokoloff L, Reivich M, Kennedy C, Des Rosiers M H, Patlas C S, Pettigrew K D, Sakurada O, Shinohara M. J Neurochem. 1977;28:897–916. doi: 10.1111/j.1471-4159.1977.tb10649.x. [DOI] [PubMed] [Google Scholar]
- 3.Shulman R G, Blamire A M, Rothman D L, McCarthy G. Proc Natl Acad Sci USA. 1993;90:3127–3133. doi: 10.1073/pnas.90.8.3127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bandettini P A, Wong E C, Hinks R S, Tikofsky R S, Hyde J S. Magn Reson Med. 1992;25:390–397. doi: 10.1002/mrm.1910250220. [DOI] [PubMed] [Google Scholar]
- 5.Raichle M E. Proc Natl Acad Sci USA. 1998;95:765–772. doi: 10.1073/pnas.95.3.765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Posner M I, Raichle M E. Images of Mind. New York: Freeman; 1994. [Google Scholar]
- 7.Smith E E, Jonides J. Cognit Psychol. 1997;33:5–42. doi: 10.1006/cogp.1997.0658. [DOI] [PubMed] [Google Scholar]
- 8.Sibson N R, Dhankhar A, Mason G F, Behar K L, Rothman D L, Shulman R G. Proc Natl Acad Sci USA. 1997;94:2699–2704. doi: 10.1073/pnas.94.6.2699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sibson N R, Dhankhar A, Mason G F, Rothman D L, Behar K L, Shulman R G. Proc Natl Acad Sci USA. 1998;95:316–321. doi: 10.1073/pnas.95.1.316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sibson, N. R., Shen, J., Mason, G. F., Rothman, D. L, Behar, K. L. & Shulman, R. G. (1998) Dev. Neurosci., in press. [DOI] [PubMed]
- 11.Magistretti P J, Pellerin L. Cereb Cortex. 1996;6:50–61. doi: 10.1093/cercor/6.1.50. [DOI] [PubMed] [Google Scholar]
- 12.Siesjo B K. Brain Energy Metabolism. New York: Wiley; 1978. pp. 358–360. [Google Scholar]
- 13.Fox P I, Raichle M E, Mintun M A, Dence C. Science. 1988;241:462–464. doi: 10.1126/science.3260686. [DOI] [PubMed] [Google Scholar]
- 14.Gjedde A. In: Cardiovascular Disease. Batjer, Hurt H, editors. Philadelphia: Lippincott; 1997. pp. 23–40. [Google Scholar]
- 15.Reivich M, Kuhl D, Wolf A, Greenberg J, Phelps M, Ido T, Casella V, Fowler J, Hoffman E, Alari A, et al. Circ Res. 1979;44:127–137. doi: 10.1161/01.res.44.1.127. [DOI] [PubMed] [Google Scholar]
- 16.Frackowiak R S J, Lenzi G L, Jones T, Heather J D. J Comput Assist Tomogr. 1980;4:727–736. doi: 10.1097/00004728-198012000-00001. [DOI] [PubMed] [Google Scholar]
- 17.Lassen N A, Ingvar D H, Shinhoj E. Sci Am. 1978;239:62–71. doi: 10.1038/scientificamerican1078-62. [DOI] [PubMed] [Google Scholar]
- 18.Ogawa S, Lee T M, Kay A R, Tank D W. Proc Natl Acad Sci USA. 1990;87:9868–9872. doi: 10.1073/pnas.87.24.9868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kwong K K, Belliveau J W, Chesler D A, Goldberg I E, Weisskoff R M, Poncelet B P, Kennedy D N, Hoppel B E, Cohen M S, Turner R, et al. Proc Natl Acad Sci USA. 1992;89:5675–5679. doi: 10.1073/pnas.89.12.5675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ogawa S, Menon R S, Tank D W, Kim S G, Merkle H, Ellerman J M, Ugurbil K. Biophys J. 1993;64:803–812. doi: 10.1016/S0006-3495(93)81441-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shepherd G M. Neurobiology. 3rd Ed. New York: Oxford Univ. Press; 1994. [Google Scholar]
- 22.Van den Berg C J, Garfinkel D. Biochem J. 1971;123:211–218. doi: 10.1042/bj1230211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hertz L, Kvamme E, McGeer E G, Schousboe A, editors. Glutamine, Glutamate and GABA in the Central Nervous System. New York: Liss; 1983. [Google Scholar]
- 24.Erecinska M, Silver I A. Prog Neurobiol. 1990;35:245–296. doi: 10.1016/0301-0082(90)90013-7. [DOI] [PubMed] [Google Scholar]
- 25.Lai J C K, Murthy Ch R K, Cooper A J L, Hertz E, Hertz L. Neurochem Res. 1989;14:377–389. doi: 10.1007/BF01000042. [DOI] [PubMed] [Google Scholar]
- 26.Norenberg M D, Martinez-Hernandez A. Brain Res. 1979;161:303–310. doi: 10.1016/0006-8993(79)90071-4. [DOI] [PubMed] [Google Scholar]
- 27.Badar-Goffer R S, Bachelard H S, Morris P G. Biochem J. 1990;266:133–139. doi: 10.1042/bj2660133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bachelard, H. S., Morris, P. G. & Taylor, A. (1994) J. Neurochem. 63, Suppl. 1, S48.
- 29.Hyder F, Chase J R, Behar K L, Mason G F, Siddeek M, Rothman D L, Shulman R G. Proc Natl Acad Sci USA. 1996;93:7612–7617. doi: 10.1073/pnas.93.15.7612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rothman D L, Novotny E J, Shulman G I, Howseman A M, Petroff O A C, Mason G F, Nixon T W, Hanstock C C, Prichard J W, Shulman R G. Proc Natl Acad Sci USA. 1992;89:9603–9606. doi: 10.1073/pnas.89.20.9603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fitzpatrick S M, Hetherington H P, Behar K L, Shulman R G. J Cereb Blood Flow Metab. 1990;10:170–179. doi: 10.1038/jcbfm.1990.32. [DOI] [PubMed] [Google Scholar]
- 32.Gruetter R, Novotny E J, Boulware SD, Mason G F, Rothman D L, Shulman G I, Prichard J W, Shulman R G. J Neurochem. 1994;63:1377–1385. doi: 10.1046/j.1471-4159.1994.63041377.x. [DOI] [PubMed] [Google Scholar]
- 33.Mason G F, Gruetter R, Rothman D L, Behar K L, Shulman R G, Novotny E J. J Cereb Blood Flow Metab. 1995;15:12–25. doi: 10.1038/jcbfm.1995.2. [DOI] [PubMed] [Google Scholar]
- 34.Shulman R G. J Cognit Neurosci. 1996;8:474–480. doi: 10.1162/jocn.1996.8.5.474. [DOI] [PubMed] [Google Scholar]
- 35.Smith E E, Jonides J, Marshuetz C, Koeppe R N. Proc Natl Acad Sci USA. 1998;95:876–882. doi: 10.1073/pnas.95.3.876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Changeux J-P. Neuronal Man: The Biology of Mind (trans. Garey, L.) (1997) Princeton: Princeton Univ. Press; 1985. [Google Scholar]
- 37.Searle J R. The Rediscovery of the Mind. Cambridge, MA: MIT Press; 1992. p. 3. [Google Scholar]
- 38.Shulman G L, Fiez J A, Corbetta M, Buckner R L, Miezin F M, Raichle M E, Petersen S E. J Cognit Neurosci. 1997;9:648–663. doi: 10.1162/jocn.1997.9.5.648. [DOI] [PubMed] [Google Scholar]
- 39.Petroff O A C, Behar K L, Mattson R H, Rothman D L. J Neurochem. 1992;67:2399–2404. doi: 10.1046/j.1471-4159.1996.67062399.x. [DOI] [PubMed] [Google Scholar]
- 40.Crick F H C. The Astonishing Hypothesis: The Scientific Search for the Soul. New York: C. Scribner’s; 1994. p. 7. [Google Scholar]
- 41.Marrett, et al. Quantification of Brain Function: Tracer Kinetics and Image Analysis in Brain PET. Amsterdam: Elsevier; 1993. pp. 217–224. [Google Scholar]
- 42.Davis T L, Kwong K K, Weisskopf R M, Rosen B R. Proc Natl Acad Sci USA. 1998;95:1834–1839. doi: 10.1073/pnas.95.4.1834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chen W, Novotny E J, Zhu X-H, Rothman D L, Shulman R G. Proc Natl Acad Sci USA. 1993;90:9896–9900. doi: 10.1073/pnas.90.21.9896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Reivich, M., Alavi, A. & Gur, R. C. (1984) Ann. Neurol. 15, Suppl., S61–S65. [DOI] [PubMed]
- 45.Roland P E, Eriksson L, Stone-Elander S, Widen L. J Neurosci. 1987;7:2373–2389. [PMC free article] [PubMed] [Google Scholar]
- 46.Borgstrom L, Chapman G, Siesjo BK. J Neurochem. 1976;27:971–973. doi: 10.1111/j.1471-4159.1976.tb05165.x. [DOI] [PubMed] [Google Scholar]