Abstract
In eukaryotic cells, DNA replication is carried out by coordinated actions of many proteins, including DNA polymerase δ (pol δ), replication factor C (RFC), proliferating cell nuclear antigen (PCNA) and replication protein A. Here we describe dynamic properties of these proteins in the elongation step on a single-stranded M13 template, providing evidence that pol δ has a distributive nature over the 7 kb of the M13 template, repeating a frequent dissociation–association cycle at growing 3′-hydroxyl ends. Some PCNA could remain at the primer terminus during this cycle, while the remainder slides out of the primer terminus or is unloaded once pol δ has dissociated. RFC remains around the primer terminus through the elongation phase, and could probably hold PCNA from which pol δ has detached, or reload PCNA from solution to restart DNA synthesis. Furthermore, we suggest that a subunit of pol δ, POLD3, plays a crucial role in the efficient recycling of PCNA during dissociation–association cycles of pol δ. Based on these observations, we propose a model for dynamic processes in elongation complexes.
INTRODUCTION
In eukaryotic cells, DNA replication is carried out by coordinated actions of many proteins. It has been demonstrated that the elongation process can be reconstituted with distinct protein factors, DNA polymerase δ (pol δ), replication factor C (RFC), proliferating cell nuclear antigen (PCNA) and replication protein A (RPA) (1–3). These replication factors, in addition to the polymerase α-primase complex (pol α), are required components of the in vitro simian virus 40 (SV40) replication system (4). The pol δ heterotetrameric complex, consisting of 125, 66, 50 and 12 kDa proteins (5), is the major polymerase involved in chromosomal replication in eukaryotic cells. RFC is composed of one large subunit (145 kDa) and four smaller ones (40, 38, 37 and 36 kDa) (6,7) and has DNA-dependent ATPase activity, loading sliding clamp PCNA onto DNA (6,8). PCNA itself is a homotrimeric ring-shaped protein with a molecular mass of 29 kDa for each monomer, which encircles double-stranded DNA (9,10). The likely role of PCNA in pol δ replication is in stabilizing the pol δ–DNA interaction to maintain the processivity of the polymerase (11–17). RPA is a heterotrimeric single-stranded (ss) DNA-binding protein, consisting of 70, 32 and 14 kDa proteins, required for the initiation and elongation phases of DNA replication (18).
The initiation phase of DNA replication with these protein factors has been investigated intensively, and it has been demonstrated that their actions are well coordinated (19–26). An important process is the switch from pol α to pol δ (24), mediated by RFC (20,22,24,26).
After an elongation complex is once established, concerted actions of these protein components could still be required. However, the architecture and actions within elongation complexes remain obscure. It is generally believed that an elongation complex containing pol δ and PCNA, but not RFC, is somehow extremely stable (27). However, we have obtained surprising results, revealing a markedly dynamic picture for elongation complexes consisting of pol δ, PCNA and RFC. We thus have evidence that pol δ frequently repeats a dissociation–association cycle at growing 3′-hydroxyl ends. RFC appears to remain at the primer terminus throughout the elongation phase, probably holding PCNA from which pol δ has detached, with the potential to catalyze unloading of PCNA. Once pol δ dissociates from a growing 3′-hydroxyl end, a significant fraction of PCNA may remain at the primer terminus through interaction with RFC, while the remainder may be unloaded by RFC or slide out of the primer terminus. RFC persisting around primer termini then may reload PCNA from solution to restart DNA synthesis. In addition, characterization of the dynamic properties of the same protein factors with a subassembly of pol δ lacking the POLD3 subunit revealed a crucial function of POLD3 in the efficient recycling of PCNA.
MATERIALS AND METHODS
Plasmids
The expression plasmid for human PCNA was as described earlier (28) and that for human RPA, p11d-tRPA (29), was a generous gift of Dr Marc S. Wold (University of Iowa College of Medicine, Iowa City, Iowa). Human cDNAs for pol δ and RFC were amplified from a HeLa cDNA library introducing a cutting site of NdeI at the start codon. The expression plasmids are listed in Supplementary Table 1, and details for their construction are described in the Supplementary Data.
Proteins
All proteins used in this study were overproduced in Escherichia coli cells (Supplementary Table 1) and purified by conventional column chromatography. During all purification steps, induced proteins were monitored by SDS–PAGE followed by staining with Coomassie Brilliant Blue R-250 (CBB) or western blotting. Protein concentrations were determined by Bio-Rad protein assay using BSA (Bio-Rad) as the standard. The procedures are described in the Supplementary Data.
Pol δ holoenzyme assays on single-stranded (ss) mp18 DNA
DNA polymerase activity was measured with reference to incorporation of [α-32P]dTMP. The standard reaction mixture (25 μl) contained 20 mM HEPES–NaOH (pH 7.5), 50 mM NaCl, 0.2 mg/ml BSA, 1 mM DTT, 10 mM MgCl2, 1 mM ATP, 0.1 mM each of dGTP, dATP, dCTP and [α-32P]dTTP, 33 fmol (240 pmol for nucleotides) of singly primed ss mp18 DNA (the 36-mer primer, CAGGGTTTTCCCAGTCACGACGTTGTAAAACGACGG is complementary to 6330–6295 nt) (30), 1.0 μg (9.1 pmol) of RPA, 86 ng (1.0 pmol as a trimer) of PCNA, 75 ng (260 fmol) of RFC and 90 ng (380 fmol) of pol δ. In the reactions with HincII, 10 U of the enzyme (Takara Bio Inc.) were introduced into the standard reaction mixture. Reaction mixtures lacking pol δ were pre-incubated at 30°C for 1 min, then reactions were started by addition of pol δ. After incubation at 30°C for 10 min, reactions were terminated with 2 μl of 300 mM EDTA, and the mixtures were immediately chilled on ice. Five-microliter samples were spotted on DE81 paper (Whatman), which was washed three times with 0.5 M Na2HPO4. The amount of incorporated [α-32P]dTMP was determined as the radioactivity retained on the paper (31). For electrophoretic analysis of replication products, 5 μl samples were mixed with 1 μl of loading buffer (150 mM NaOH/10 mM EDTA/6% sucrose/0.1% bromophenol blue), and electrophoresed on 0.7% alkaline-agarose gels as described (32). To detect the products by Southern blotting, the newly synthesized strands were hybridized with a 5′-32P-labeled oligonucleotide (GCACCCCAGGCTTTACACTTTATGCTTCCGGCTCGTATGTTGTGTGGAATT GTGAGCGGATAACAATTTCACACAGGAAACAGCTATGACCATGATTAC). For linearization of ss mp18 DNA before reactions, an oligonucleotide, CAGGGTTTTCCCAGTCACGACGTTGTAAAACGACGGCCAGTGCCAAGCTTGCA TGCCTGCAGGTCGACTCTAGAGGATCCCCGGGTACCGAGCTCGAATT, was annealed to the template.
Isolation of PCNA on DNA bound to magnetic beads
The 5′-biotinylated primer (CTCTCTCTCTCTCTCTCTCTCAGGGTTTTCCCAGTCACGACGTTGTAAAACGACGG) was annealed to 33 fmol ss mp18 DNA, immobilized onto a 15-μl suspension of streptavidin magnetic beads, M280 (Dynabeads) in 20 mM HEPES–NaOH (pH 7.5), 50 mM NaCl, 0.2 mg/ml BSA, 1 mM DTT by incubation at room temperature for 30 min and chilled on ice. The buffer condition of the mixture was adjusted to that of the pol δ standard assay. For the reaction with HincII, 10 U of the enzyme (Takara Bio Inc.) were introduced into the standard reaction mixture. Reaction mixtures lacking pol δ were pre-incubated at 30°C for 1 min, then pol δ or buffer (as control) was introduced into the reaction mixture. After incubation at 30°C for 10 min, reactions were terminated with 2 μl of 300 mM EDTA, and then the mixtures were immediately chilled on ice. Subsequent washes were carried out at 4°C using a Dynal magnet with 40 μl of wash buffer [20 mM HEPES–NaOH (pH 7.5), 0.5 M NaCl, 0.2 mg/ml BSA, 1 mM DTT, 1 mM EDTA] four times. The beads were boiled in sample loading buffer and proteins were separated on a SDS 4–20% gradient polyacrylamide gel, blotted onto a nitrocellulose membrane, and probed with an anti-PCNA antibody (Santa Cruz, sc-7907). Detection was achieved with an ECL chemiluminescence kit (GE Healthcare Life Science). Different exposures of the blot were photographed with a CCD camera and quantified.
RESULTS
Reconstitution of DNA synthesis
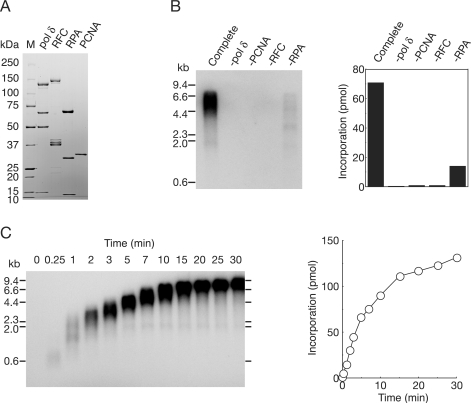
We initially tried to establish procedures to purify the replication proteins (pol δ, RFC, PCNA and RPA), at quantities sufficient for detailed biochemical studies. Because it has been shown that bacterial systems are very powerful for large-scale production of PCNA and RPA as complexes (24,28,29), we developed expression systems for heterotetrameric pol δ and heteropentameric RFC in E. coli and the complexes were then purified by conventional column chromatography (Figure 1A).
Figure 1.
Reconstitution of DNA replication with recombinant replication factors on singly primed ss mp18 DNA. (A) SDS–PAGE analysis of purified recombinant proteins. Pol δ (2.4 μg), RFC (1.5 μg), RPA (1.2 μg) and PCNA (0.8 μg) were loaded on a SDS 4–20% gradient polyacrylamide gel and stained with CBB. (B) Requirement of replication factors for synthesis of singly primed ss mp18 DNA. Reactions were carried out for 10 min under the conditions described in the Materials and Methods section or omitting one replication factor. Products were analyzed by 0.7% alkaline-agarose gel electrophoresis as described in the Materials and Methods section. Incorporation of dNMP was measured as described in the Materials and Methods section. (C) Time course of the reaction of DNA synthesis. The reaction products were analyzed by the same procedures as for (B).
First, we measured activities of purified proteins on singly primed ss mp18 DNA. In this assay, all of the protein components, RPA, PCNA and RFC, were found to be required for maximum activity of pol δ (Figure 1B) (6,33,34). In the reaction for 10 min, we detected a long product, probably corresponding to the full-size mp18 DNA (7.2 kb) (Figure 1B), and the amount increased with further incubation (Figure 1C). The size of the products was heterogeneous, indicating several pausing sites. Omitting PCNA or RFC from the reaction mixture resulted in virtually no incorporation of dNMP (Figure 1B). In the absence of RPA, heterogeneity in the length of the products was emphasized (Figure 1B) (6,34,35). Under the experimental conditions used, efficient dNMP incorporation was observed within the first 5 min, and then DNA synthesis continued at slower rates (Figure 1C). These results indicated that the recombinant proteins efficiently interacted with each other in the holoenzyme assay.
Differential modes of action of protein components on mp18 replication
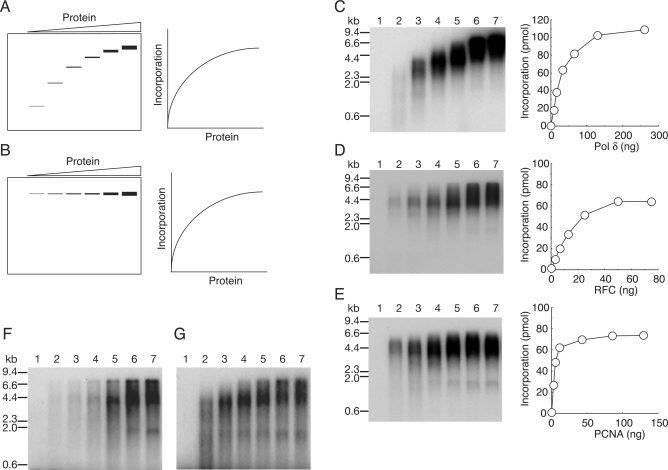
Theoretically, proteins act during replication in two distinct modes (32). Some proteins remain associated continuously with the growing 3′-hydroxyl end (acting processively), and others repeat a cycle of association and dissociation (acting distributively). The distributive factors have to be reloaded many times from solution during elongation, while the processive factors are loaded only once at the initiation.
How each of the proteins acts during replication on ss mp18 DNA can be investigated (32) as described below. If a protein acts distributively, then the product size is expected to vary proportionally with the concentration of the protein in the reaction mixture; the average size of products should be shorter at lower concentrations of the proteins because association of the protein to primer termini would be random and all primer termini would be utilized with the same efficiency (Figure 2A). On the other hand, if a protein acts processively, limiting its concentration in solution would be expected to affect only the frequency of the first assembly event (initiation), but not the size of products (Figure 2B). If the association and dissociation rates were much faster than other chemical steps, the binding process would not be rate limiting, and the incorporation rate of dNMP would not vary (Figure 2A and B, right panels). In the following experiments, we investigated the mode of action of proteins by analyzing size distributions of products by electrophoresis on agarose gels under denaturing conditions.
Figure 2.
Analysis of the modes of action of replication factors in ss mp18 DNA replication. (A and B) Expected results of titration of a distributive factor (A) or a processive factor (B) on the DNA replication. The left panels represent gel images of alkaline-agarose gel electrophoresis, and the right panels represent graphs of quantified data for reaction products. See the text for details. (C–E) Titration of replication factors, pol δ (C), RFC (D) and PCNA (E). Reactions were carried out for 10 min under the conditions described in the Materials and Methods section except for variation in the amounts of single protein factors. Reaction products were analyzed by 0.7% alkaline-agarose gel electrophoresis and the newly synthesized DNA were visualized by the incorporated [α-32P]dTMP (left panels). Incorporation of dNMP were measured as described in the Materials and Methods’ section (right panels). Titration of pol δ (C); 0 ng (lane 1), 8.1 ng (lane 2), 16 ng (lane 3), 33 ng (lane 4), 65 ng (lane 5), 130 ng (lane 6) and 260 ng (lane 7). Titration of RFC (D); 0 ng (lane 1), 3.1 ng (lane 2), 6.3 ng (lane 3), 13 ng (lane 4), 25 ng (lane 5), 50 ng (lane 6) and 75 ng (lane 7). Titration of PCNA (E); 0 ng (lane 1), 2.7 ng (lane 2), 5.4 ng (lane 3), 11 ng (lane 4), 43 ng (lane 5), 86 ng (lane 6) and 129 ng (lane 7). (F and G) Titration of RFC (F) and PCNA (G) in the reactions using the 5′-32P-labeled primer. The labeled primer was annealed to ss mp18 DNA, and [α-32P] dTTP was replaced with cold dTTP in the reaction mixtures. Amounts of proteins used in the titration were the same as for D and E.
First, the concentration of pol δ was varied in the presence of saturating amounts of the auxiliary proteins, and the product size was analyzed by alkaline-agarose gel electrophoresis (Figure 2C). The results showed that the amount and length of the products increased as more pol δ was added to the reaction mixture (Figure 2C), implying that pol δ frequently dissociated from the growing 3′-hydroxyl end and reassociated randomly with free 3′-hydroxyl ends (34–36). The dissociation might be caused by the secondary template structure (35). Therefore, we conclude that pol δ is not completely processive in this assay system.
Next, we changed concentrations of RFC (Figure 2D). The result was different from the case of pol δ. Essentially, the concentration of RFC affected only the amount of products, but not their size (Figure 2D) (6,37), although increase of size at higher concentrations of RFC was noted (Figure 2D, lanes 6 and 7). The processive nature of RFC might be explained in two alternative ways. One is that the sole role of RFC is loading of PCNA at the initiation step (38). Once PCNA is loaded, due to stable association with DNA, RFC may no longer be required. If so, even after dissociation of pol δ from the growing 3′-hydroxyl end, once loaded PCNA may remain at the primer terminus and assist reassociation of pol δ. The other explanation is that once RFC binds to a primer end and loads PCNA to DNA, it may travel with PCNA and pol δ (26,39,40). In the latter case, after dissociation of pol δ, RFC may continue to hold PCNA at the primer terminus, and if the RFC unloaded PCNA (41–43) or if PCNA slide out of the primer terminus (44), the remaining RFC could reload PCNA quickly from solution. Therefore, it seems to be a very critical question, how the concentration of PCNA affects the size of the products. If PCNA behaves as a processive factor, RFC should be required only for the initiation step to load PCNA. On the other hand, if PCNA behaves as a distributive factor, then RFC would have to be traveling with growing 3′-hydroxyl ends as a processive factor for loading PCNA anytime during elongation.
Thus, we subsequently examined effects of varying PCNA concentrations on the product size distribution. When the concentrations of PCNA were low, sizes of the products became slightly decreased and smears <2 kb appeared (Figure 2E, lanes 2–4). However, large products (4–5 kb) were also detected, even at the lowest concentration of PCNA (Figure 2E, lane 2). In these assays, full-size products are excessively represented by their greater incorporation of radioactive nucleotides. To avoid this problem, we used a 5′-32P-labeled primer. Note that the size distribution of the products in the PCNA titration, different from that in RFC titration, was more clearly visualized with a 5′-32P-labeled primer than with incorporation of [α-32P]dTMP (Figure 2F and G). The results revealed that the concentration of PCNA did not affect the initiation step, because the amount of utilized primer was not appreciably decreased with lower concentrations of PCNA (Figure 2G). On the other hand, the initiation was limited at a low concentration of RFC (Figure 2F), suggesting inefficient turnover of RFC. We consider that after dissociation of pol δ from the growing 3′-hydroxyl end, some PCNA does not remain at the primer terminus, rather sliding out or being unloaded by RFC. In both cases, another PCNA might be reloaded from solution. Therefore, the results suggested that PCNA is partially distributive in our assay system, and also suggested that the processive nature of RFC is due to a stable association at the primer terminus during elongation of DNA replication. These issues were addressed intensively in subsequent experiments.
DNA replication on linearized ss mp18 DNA
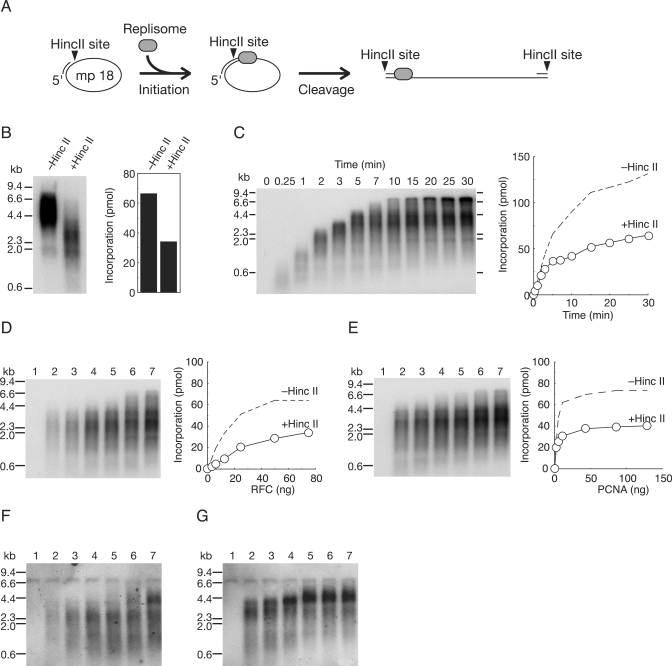
For further analysis of PCNA and RFC actions during elongation, we examined the effects of linearization of mp18 DNA after initiation of DNA replication by introduction of a restriction enzyme, HincII, into reaction mixtures (Figure 3). A unique cutting site of HincII in the template DNA is located 29-bases downstream from the 3′-hydroxyl end of the primer. Soon after initiation of DNA replication, the region should be converted to double-stranded DNA and become cleavable by HincII (Figure 3A). In standard reaction mixtures, we introduced 10 U of HincII that can digest more than 98% of double-stranded mp18 DNA, for 15 s. As shown in Figure 3B, the sizes of all the products became uniformly much shorter in the presence of HincII and incorporation of dNMP was decreased by half (Figure 3B), probably due to a defect in elongation, rather than initiation, because the average size of products was also reduced by almost a half (Figure 3B). We considered that the decreased efficiency of DNA synthesis was probably the consequence of dissociation of PCNA, suggesting that some PCNA once assembled into the elongation complex might not remain at primer termini during elongation, and with a circular template, the majority of detached PCNA slides back to be reutilized.
Figure 3.
Effects of linearization of newly synthesized DNA just after initiation of DNA replication. (A) Schematic representation of the experimental design. A restriction enzyme, HincII, was introduced into the reaction mixtures. A unique cutting site in the template DNA is located 29-bases downstream from the 3′-hydroxyl end of the primer. After the region was converted to double-stranded DNA by initiation of DNA synthesis, indicating assembly of elongation complexes, the DNA becomes cleavable by HincII. (B) Effects of HincII on synthesis of ss mp18 DNA. (C) Time course of DNA synthesis in the presence of HincII. (D and E) Titration of RFC (D) and PCNA (E) in the presence of HincII. Amounts of PCNA and RFC used in the titration were the same as used for Figure 2 (see legend of Figure 2). Autoradiograms of 0.7% alkaline-agarose gels (left panels) in which the newly synthesized DNA was visualized by the incorporated [α-32P]dTMP, and incorporation of dNMP were measured as described in the Materials and Methods section (right panels). Dotted lines represent results without HincII of Figure 2B. (F and G) Titration of RFC (F) and PCNA (G) in the presence of HincII. [α-32P] dTTP was replaced with cold dTTP in the reaction mixtures and the newly synthesized strands were visualized by Southern blotting with a 5′-labeled oligonucleotide, which is complementary to newly synthesized strand just downstream of HincII site. Amounts of proteins used in the titration were the same as for (D) and (E). Reactions in (B, D–G) were carried out for 10 min under the conditions described in the Materials and Methods section with addition of HincII (10 U/25 μl of reaction mixture).
The results shown in Figure 2 suggest that RFC remains at growing 3′-hydroxyl ends and reloads PCNA from solution during elongation. This implies that DNA synthesis can be resumed again after PCNA is once dissociated from the primer termini. If this were indeed the case, the size of the products should increase in a time-dependent manner. The results shown in Figure 3C indicate that incorporation increased and the products became longer depending on the incubation time, but with a slower rate, suggesting reloading of PCNA from solution. Because pausing likely promotes the dissociation of pol δ from PCNA (35), some PCNA also would be released from the primer termini at pausing sites. As expected these became more prominent, resulting in a more heterogeneous size distribution of products (Figure 3C).
Then, we further tested whether the concentration of RFC could affect the size of products in the presence of HincII (Figure 3D). Again, only the amount, but not the size distribution, was affected, although increase of the size at higher concentrations of RFC was observed, as in the reaction without HincII (Figure 2D). In contrast, the concentration of PCNA partially affected the size distribution of products (Figure 3E). The difference between RFC and PCNA titration was not clear by visualization with incorporation of the radioactive nucleotides. However, the 5′-32P-labeled primer, as shown in Figure 2F and G, was unavailable because of the restriction cutting. To solve the problem, newly synthesized strands were visualized, instead, by Southern hybridization with an oligonucleotide annealed to the newly synthesized strand just downstream of the HincII site. Results confirmed the difference in size distributions of the products between in the PCNA titration and RFC titration (Figure 3F and G). Since some PCNA slides out of the DNA (Figure 3B) and is reloaded from solution many times by RFC to continue DNA synthesis (Figure 3C), the size of the products would depend on the concentration of RFC, if this acted distributively. Therefore, our results again suggested that RFC acts processively, binding in the initiation of DNA replication and traveling with pol δ.
Inefficient elongation by spontaneous loading of PCNA from template DNA ends
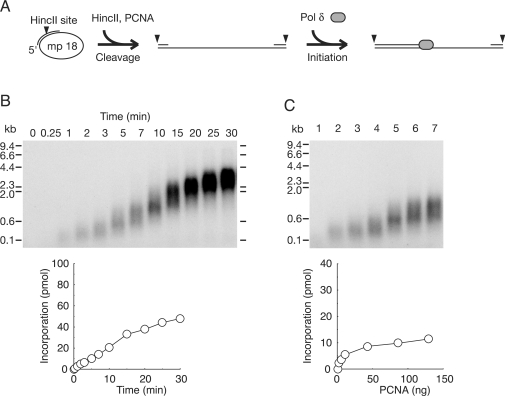
It is known that PCNA is spontaneously loaded from a double-stranded end of template DNA in an RFC-independent manner, and supports elongation with pol δ (44). Therefore, the restart reactions after dissociation of PCNA observed in Figure 3C could be due to spontaneous loading of PCNA at the ends. To determine the efficiency of RFC-independent restart, ss mp18 DNA was linearized first, then subjected to reactions in the absence of RFC. In this assay, a primer covering HincII site was annealed to ss mp18 DNA (Figure 4A). HincII was introduced in standard reaction mixtures, and then the reactions were started by addition of pol δ after pre-incubation for 1 min (Figure 4A). The time course of the reaction revealed the extension rate to be much slower (Figure 4B) than that of RFC-dependent reactions (Figure 3C) and the primer ends were hardly extended beyond the pausing site around 4 kb (Figure 4B). Next, we examined effects of varying PCNA concentrations on the size distribution of the products. The results demonstrated that the length of the products is uniformly short at a low concentration of PCNA (Figure 4C), suggesting that PCNA once assembled with pol δ is not stable during elongation. These results revealed differences between RFC-dependent and -independent reactions, indicating a requirement of RFC for efficient restart after dissociation of PCNA on linearized DNA.
Figure 4.
Effect of PCNA loaded spontaneously at ends of template DNA. (A) Schematic representation of the experimental design. A primer that covered HincII site was annealed to ss mp18 DNA. HincII (10 U) was introduced into standard reaction mixtures (25 μl) under the conditions described in the Materials and Methods section except for omitting RFC. After pre-incubation for 1 min, reactions were started by addition of pol δ. (B) Time course of DNA synthesis in the absence of RFC. (C) Titration of PCNA in the reactions without RFC. Amounts of PCNA used in the titration were the same as for Figure 2 (see legend of Figure 2). Reactions were carried out for 10 min. Autoradiograms of 0.7% alkaline-agarose gels in which the newly synthesized DNA were visualized by the incorporated [α-32P]dTMP, and incorporation of dNMP were measured as described in the Materials and Methods section.
Effects of dilution of the elongation complexes
A different method of analysis was used to investigate the action of protein factors. Here, after the replication complex was assembled, the reaction mixture containing the complexes was diluted in pre-warmed reaction mixtures containing all the protein components at the same concentration, except one component. As with the previously described method, if the protein in question acts distributively, one would expect to observe a shift to shorter products with diluted reaction mixtures (32).
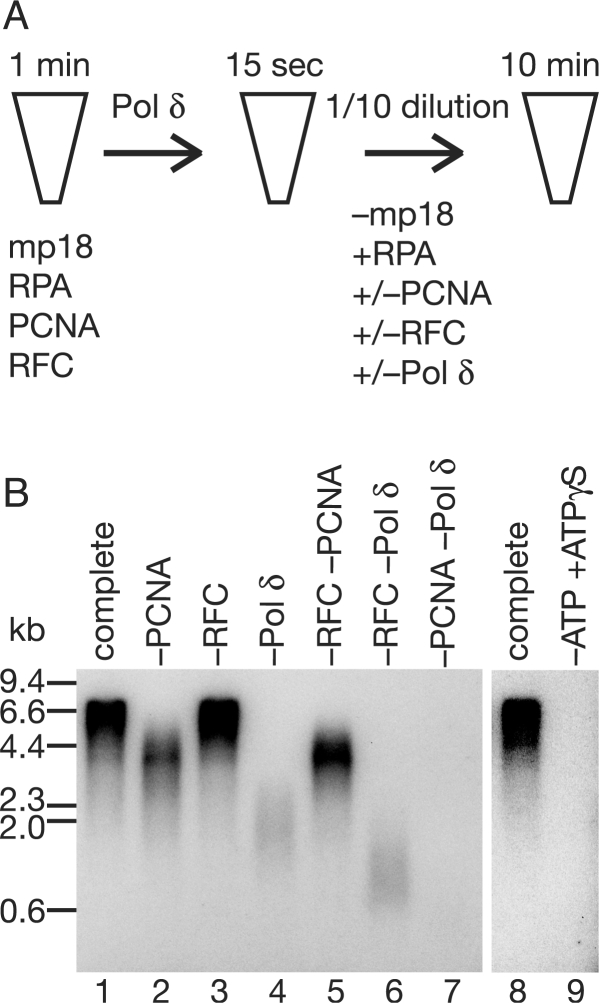
As schematically shown in Figure 5A, the primer–template DNA was mixed with saturating concentrations of RPA, PCNA and RFC, and then 1 min later pol δ was added for formation of replication complexes. At 15 s after the initiation complex was presumably assembled, an aliquot of the reaction mixture was diluted 10-fold either into a pre-warmed reaction mixture containing all the auxiliary proteins and pol δ, or into a similar one omitting one or two of PCNA, RFC and pol δ (Figure 5A). Then, reactions were continued for further 10 min, and the products were analyzed by alkaline-agarose gel electrophoresis (Figure 5B). Dilution of either PCNA or pol δ resulted in a decrease in the size of the products (Figure 5B, lanes 2 and 4) as compared to the complete case (Figure 5B, lane 1). This effect was more pronounced when both PCNA and pol δ were diluted together (Figure 5B, lane 7); virtually no products were detected. On the other hand, dilution of RFC exerted no influence (compare lanes 1 and 3 in Figure 5B). Furthermore, when both PCNA and RFC were diluted together, the size distribution of products was almost identical to that with dilution of PCNA alone (compare lanes 2 and 5 in Figure 5B). These results indicated that PCNA and pol δ are supplied from solution, whereas RFC is not, during the elongation. Furthermore, we noted that when both RFC and pol δ were diluted together, the product size was decreased to a much greater extent than with dilution of pol δ alone (compare lanes 4 and 6 in Figure 5B). This suggested that when reassociation of pol δ is limited, RFC is needed from solution. The importance of this observation is discussed below.
Figure 5.
Effects on size distribution after dilution of elongation complexes. (A) Outline of the assay. At 15 s after the reaction was started by addition of pol δ under standard reaction conditions described in the Materials and methods section, 10-fold dilution was performed with pre-warmed reaction mixtures without template but containing all the protein components or omitting one or two of them. Both reaction mixtures, before and after dilution, contained [α-32P]dTTP. After a further 10 min incubation, the reaction products were analyzed by 0.7% alkaline-agarose gel electrophoresis. (B) An autoradiogram of a 0.7% alkaline-agarose gel. The indicated proteins were omitted in the dilution mixtures. In the reaction shown in lane 9, 1 mM ATP in the dilution mixture was replaced with 2.5 mM ATPγS.
We also tested if adenosine (3-thiotriphosphate) (ATPγS) affected elongation reactions. When ATP in the dilution mixture was replaced with ATPγS, elongation reactions were completely halted (Figure 5B, lane 9). This suggested that the ATPase activity of RFC is required for the elongation phase of replication (24), consistent with our assumption that RFC remains around primer terminus, and holds, unloads and reloads PCNA.
Functions of the POLD3 subunit of pol δ
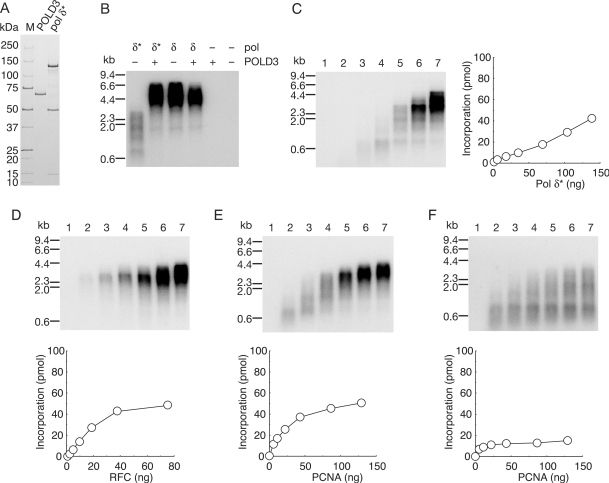
The role of the 66 kDa subunit, POLD3, of pol δ in the dynamic processes involved in elongation, and the biochemical properties of subassembly (pol δ*) lacking the POLD3 subunit are of great interest. First, we examined the efficiency of DNA synthesis of human pol δ* using purified proteins (Figure 6A). A comparison of activities with equivalent amounts of pol δ* and pol δ demonstrated inefficiency of pol δ* under the standard reaction conditions with singly primed ss mp18 DNA (Figure 6B), decrease and heterogeneity in length of the products being observed with emphasized pausing sites (Figure 6B and C). The shorter products were shifted to longer ones at higher concentrations of pol δ* (Figure 6C), as with pol δ (Figure 2C). When the missing subunit, POLD3, was introduced into the reaction with pol δ*, the activity was restored to the level with pol δ (Figure 6B), indicating that the lower activity of pol δ* was due to the missing function of POLD3 subunit, rather than denaturation of proteins caused by incomplete assembly. The evidence presented here is consistent with reports for yeast counterparts (30,45) and human pol δ (36,46).
Figure 6.
Dynamics of replication factors in the elongation phase of DNA synthesis with pol δ*. (A) SDS–PAGE analysis of purified recombinant proteins. Pol δ* (1.9 μg) and POLD3 (0.5 μg) were loaded on a SDS 4–20% gradient polyacrylamide gel and stained with CBB. (B) Reconstitution of pol δ with POLD3 and pol δ*. Reactions were carried out for 10 min under the conditions described in the Materials and Methods section except for pol δ* (70 ng) or pol δ (90 ng) in the absence (−) or presence (+) of POLD3 (20 ng). (C–E) Titration of pol δ* (C), RFC (D) and PCNA (E). Amounts of pol δ* were 0 ng (lane 1), 4.3 ng (lane 2), 17 ng (lane 3), 35 ng (lane 4), 70 ng (lane 5), 100 ng (lane 6), and 140 ng (lane 7). Amounts of RFC used in the titration were 0 ng (lane 1), 2.3 ng (lane 2), 4.7 ng, (lane 3), 9.4 ng (lane 4), 19 ng (lane 5), 38 ng (lane 6) and 75 ng (lane 7). Amounts of PCNA used in titration were 0 ng (lane 1), 5.4 ng (lane 2), 11 ng (lane 3), 22 ng (lane 4), 43 ng (lane 5), 86 ng (lane 6) and 129 ng (lane 7). (F) Titration of PCNA in the presence of HincII. Amount of PCNA is same as (E). Reactions in (C) were carried out for 10 min under the conditions described in the Materials and Methods section. Reactions in (D–F) were carried out for 10 min under the conditions described in the Materials and Methods section except for the amount of pol δ* (140 ng). Products were analyzed by 0.7% alkaline-agarose gel electrophoresis and incorporation of dNMP were measured as described.
Next, we tested whether variation in the concentrations of RFC and PCNA might affect the size of products in reactions with pol δ*. RFC was without influence, again suggesting stable association (Figure 6D). In contrast, the size of products varied with the concentration of PCNA (Figure 6E), in line with a requirement for a continuous supply of PCNA from solution for efficient DNA synthesis. Notably, with low concentrations of PCNA, all the products were uniformly small in reactions with pol δ*, exhibiting an entirely distributive nature. Furthermore, we tested the effect of linearization on mp18 DNA after initiation of DNA replication by addition of HincII (Figure 6F). DNA synthesis with pol δ* was very sensitive to linearization of DNA, and increasing concentrations of PCNA slightly restored the defect (Figure 6F). However, the majority of intermediates on elongation could not overcome the first pausing site (around 0.6–1 kb), even at the highest concentration of PCNA (Figure 6F). These results suggested frequent dissociation of PCNA during elongation with pol δ*.
Amounts of PCNA loaded on mp18 DNA during elongation
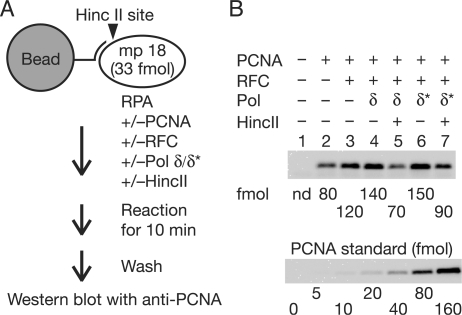
Since an excess PCNA was required for efficient DNA synthesis with pol δ* (Figure 6E), we considered whether PCNA might accumulate on DNA during elongation. If so, the frequency of sliding back to the primer terminus would increase and an increase in the local concentration of PCNA would help interactions with pol δ* at the primer terminus. To measure the amount of PCNA loaded on DNA directly, a primer containing an extended 5′ tail with one biotin molecule was annealed to ss mp18 DNA (Figure 7A). The primed ss mp18 DNA was then attached to magnetic beads and DNA replication reactions were carried out under standard reaction conditions (Figure 7A). The 5′ tail did not exert any influence on DNA synthesis (data not shown). In the reactions, 33 ng of pol δ and 140 ng of pol δ* were used since these amounts lead to equivalent efficiency of DNA synthesis (∼40 pmol of the incorporation of dNMP) and to the same size distribution of products (compare lane 3 of Figure 2C with lane 7 of Figure 6C). After reactions for 10 min, PCNA bound to beads was detected by western analysis (Figure 7B). In this assay, non-specific association of PCNA with beads or DNA was detected (Figure 7B, lane 2). When RFC was introduced into the reaction, an increase of binding of PCNA was observed. The increased amount of PCNA (difference between lanes 2 and 3 in Figure 7B) was 40 fmol, which was equivalent to that of primer template (33 fmol), suggesting specific loading to the primer terminus. Introduction of pol δ increased the amount of PCNA only slightly (Figure 7B, lane 4). The majority of PCNA was dissociated by introduction of HincII with a decreasing signal to background level (Figure 7B, lane 5), again indicating specific loading on the DNA. When pol δ* was used instead of pol δ, excessive accumulation of PCNA was unexpectedly not observed (Figure 7B, lane 6), suggesting that we cannot attribute the entire distributive nature of PCNA on the reaction with pol δ* to only the decreasing affinity between PCNA and pol δ*. We therefore considered the possibility that loading and unloading of PCNA is equilibrated in both pol δ and pol δ* cases, and importantly, could be accelerated in the reaction with pol δ*.
Figure 7.
Amounts of PCNA loaded on DNA during elongation. (A) Outline of the assay. DNA was attached to magnetic beads via biotin–streptavidin linkage. The reactions were carried out for 10 min under the conditions described in the Materials and Methods section except for the amounts of pol δ (33 ng) and pol δ* (140 ng). (B) Western analysis. Chemiluminescence signals were detected with a CCD camera and quantified with reference to a standard curve for PCNA in the same blot.
DISCUSSION
Generally, proteins act during replication in two distinct modes, processively or distributively (32). The studies documented here showed very different dynamics of the various protein factors in the elongation phase of DNA replication.
DNA synthesis in vitro by pol δ has been investigated extensively. Previous studies have shown that pol δ itself is a very distributive enzyme, which turns into a processive polymerase when bound to the clamp, PCNA (11,12,15–17). However, even in the presence of PCNA, pol δ replicated M13 ss DNA through a number of dissociating and reassociating steps, as proposed for mammalian pol δ isolated from natural sources and overproduced in insect cells (34–36). Our results support the conclusion that human pol δ has a distributive nature for DNA replication in our model system in vitro.
The length of products synthesized by pol δ does not depend on the concentration of RFC (6,37). The processive nature of RFC might be explained in two alternative ways. One is that the sole role is loading of PCNA at only the initiation step (35,38). Some biochemical data for yeast and human RFC support this possibility, because RFC has been found to dissociate from DNA after loading PCNA (38,47,48). The other explanation is that once RFC finds a 3′-hydroxyl end and loads PCNA, it then travels with PCNA and pol δ (39,40). In earlier work with yeast RFC, formation of tertiary complexes on DNA was also suggested (49). Later, Yuzhakov and colleagues (26) also reported that human RFC travels with pol δ, and a similar complex has been isolated from the elongation phase of SV40 replication (50). Our observations also provide support for continuous binding of RFC.
Because PCNA is a sliding clamp, it should be able to freely slide along double-stranded DNA and fall off at the ends (43,44,51). However, the fate of PCNA in the elongation phase of DNA replication is currently obscure. We here obtained evidence that some PCNA does not remain at the primer terminus after dissociation of pol δ. However, in titration experiments of PCNA, the longer products (around 4 kb) still remained even at low concentrations of PCNA, independent of the presence or absence of HincII (Figures 2E and G, 3E and G), indicating that significant fractions of the intermediates of elongation phase could continue DNA synthesis without supply of PCNA from solution. In such fractions, PCNA must remain at the primer terminus even after dissociation of pol δ. The observations support the conclusion that PCNA has a partially distributive nature in our DNA replication system with RFC in vitro. On the other hand, PCNA was entirely distributive in the RFC-independent reaction (Figure 4C). Taken the results together, we suggest the possibility that RFC could hold PCNA from which pol δ has detached.
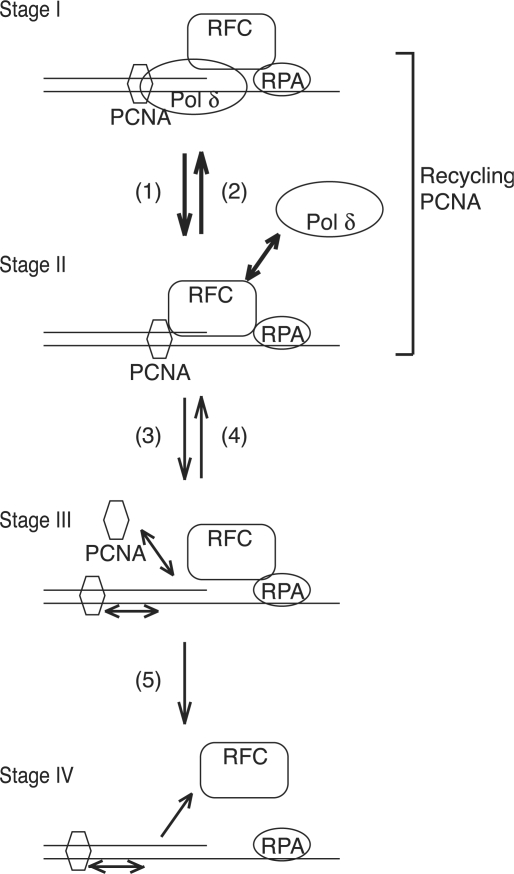
We here propose a model for dynamics of replication factors during a dissociation–association cycle of pol δ (Figure 8). The elongation complex consists of RFC, PCNA and pol δ (Figure 8, stage I). Pol δ dissociates frequently from growing 3′-hydroxyl ends and PCNA during elongation of DNA replication (Figure 8, pathway 1). Then, PCNA from which pol δ has detached would be held by the remaining RFC (Figure 8, stage II). If pol δ reassociated quickly (Figure 8, pathway 2), RFC could remain around the primer terminus, and travel with pol δ and PCNA during elongation of DNA replication. In this cycle, PCNA is not released out of the replication complex, which implies ‘recycling PCNA’ (Figure 8). Since the DNA–RFC–PCNA complex (stage II) is not stable (52), the RFC could unload PCNA, or release the PCNA out of the primer terminus (Figure 8, pathway 3). Probably, the unloading reaction does not predominate, as shown in yeast RFC (53). If PCNA were available from solution or along the DNA, RFC could incorporate PCNA into the complex (Figure 8, pathway 4). Otherwise, RFC would dissociate from the primer terminus (Figure 8, pathway 5). At higher concentrations greater than saturated amounts of RFC, the size of products was increased (Figures 2D and F, 3D and F). Probably, with such concentrations, RFC is sufficient for initiation and remaining RFC in solution could help to overcome the pausing site, implying dissociation of RFC at strong pausing sites. However, this was negligible under normal replication conditions, and was detectable in reactions omitting pol δ, as shown in lane 6 of Figure 5B. This suggested that pol δ prevents dissociation of RFC. In intensive studies of PCNA-loading reactions with yeast RFC, no stable RFC–DNA complex was detected in the absence of PCNA and only became detectable in the presence of ATPγS, and RFC dissociated quickly from DNA after loading PCNA (47,48). Therefore, it has been considered that RFC is absent in elongation complexes. Our results may explain the discrepancy regarding the prevention of dissociation of RFC by sequential loading of PCNA (pathway 4) and pol δ (pathway 2) in the replication assay, but not PCNA loading assays. Therefore, we consider that our model is consistent with the previous observations (38,47,48). Gomes et al. (47) has also proposed a loading pathway, in which PCNA–RFC complex first forms an ATP-dependent ring-opened complex and subsequently associates with DNA and delivers PCNA to the template–primer junction. RFC associated with the DNA cannot recruit PCNA nor load it at termini, first having to dissociate coupled with ATP hydrolysis (47). Our model is consistent with the loading mechanism. RFC is probably detached from the primer terminus before loading PCNA, but associated around primer terminus via interaction with RPA (stage III) as proposed previously (26).
Figure 8.
A model for dynamics of replication factors during pol δ dissociation–association cycles. The elongation complex consists of RFC, PCNA and pol δ in the elongation phase of DNA replication (Stage I). Pol δ contacts with PCNA and prevents RFC from dissociating. Contribution of RFC–RPA interaction for stable association in the complex has been proposed (26). Pathway 1, dissociation of pol δ leaving RFC and PCNA on DNA (Stage II). DNA–RFC–PCNA complex formation could be coupled with dissociation of pol δ, mediated by the POLD3 subunit. Pathway 2, reassociation of pol δ to form the elongation complex. The POLD3 subunit of pol δ might mediate efficient transfer of PCNA from RFC to pol δ. Pathway 3, unloading or sliding of PCNA out of the primer terminus, leaving RFC (Stage III). RFC probably interacts with RPA for retaining around primer terminus (26). Pathway 4, reloading of PCNA from solution or PCNA sliding back along the DNA to reform the DNA–RFC–PCNA complex. Pathway 5, dissociation of RFC from DNA (Stage IV). The main pathways are shown as thick arrows.
Here, we further examined the dynamics of protein factors in the reaction with pol δ* to elucidate functions of the POLD3 subunit. This, together with its budding and fission yeast counterparts, Pol32 and Cdc27, respectively, has a PCNA-binding domain that is responsible for processive DNA synthesis on M13 ss DNA (54–57). We demonstrated that the size of products varies depending on the concentration of PCNA exhibiting an entirely distributive nature (Figure 6E). If PCNA were accumulated on the DNA reflecting an increase amount in solution, the requirement of large excess of PCNA in solution could be simply due to decreasing affinity between pol δ* and PCNA. We failed to demonstrate excessive accumulation of PCNA on the DNA (Figure 7B). Rather, the concentration of PCNA in solution little affected that on DNA. Therefore, it is impossible to attribute the entire distributive nature of PCNA on the reaction with pol δ* to simply a decreasing affinity between PCNA and pol δ*. We consider that the defect with pol δ* could be due to not only decreased affinity to PCNA, but also failure in recycling of PCNA after dissociation. In the reactions with pol δ, significant fractions of intermediates in the elongation stage could continue DNA synthesis without supply of PCNA from solution (Figure 2E), indicating efficient recycling of PCNA (Figure 8, pathways 1 and 2). In contrast, such a fraction was not detected in reactions with pol δ* (Figure 6E), presumably due to predominant loss of PCNA from the primer terminus. This could be a consequence of unloading of PCNA by RFC, since excessive accumulation of PCNA was not observed (Figure 7B) under conditions whereby excess loading of PCNA from solution was expected (Figure 6E). Yeast studies have predicted a second function of subunits enhancing processivity of pol δ in a manner independent of the PCNA-binding site (56). We propose that the previously unknown function of POLD3 subunit is stimulation of recycling of PCNA in the dissociation–association cycle with pol δ.
It has been shown that RFC binds non-specifically to DNA with a potential for loading PCNA on the double-stranded DNA, independent of the primer ends (58). Is the processivity of RFC observed in this work only apparent and due to non-specific loading of PCNA? If PCNA molecules were loaded anywhere on the double-stranded DNA and accumulated on the DNA, it could explain that size of the products of DNA synthesis depends on the concentration of PCNA, especially in reactions with pol δ* (Figure 6E). However, we failed to detect excessive accumulation of PCNA on the DNA (Figure 7B). Furthermore, spontaneously loaded PCNA from the end of DNA did not well support elongation, even an excessive amount of PCNA was present in the RFC-independent reactions (Figure 4), suggesting that loading of PCNA at primer terminus is crucial for the efficiency. We speculate that PCNA loaded non-specifically on the DNA is probably ineffective, just like spontaneously loaded PCNA. Although we could not exclude the possibility of contribution of non-specific loading of PCNA to the apparent processivity of RFC, it is probably not predominant in our reaction conditions.
PCNA functions as a platform not only for elongation but also for Okazaki fragment processing through interaction with protein factors, FEN1 and DNA ligase I. Recently, the Kunkel laboratory has provided evidence that pol ε is active in DNA synthesis on the leading strand (59), suggesting the pol δ is active on the lagging strand (59,60). Consistent with this, stable association of RFC in elongation complexes with pol δ for efficient utilization of PCNA could thus be of benefit to maturation of Okazaki fragments. Consistently, physical interactions between RFC and DNA ligase I have been demonstrated (61), implying a functional significance of stable association of RFC in elongation complexes on the lagging strand.
SUPPLEMENTARY DATA
Supplementary Data are available at NAR Online.
ACKNOWLEDGEMENTS
We thank Dr Marc S. Wold (University of Iowa College of Medicine, Iowa City, Iowa, USA) and Dr Tadashi Shimamoto (Hiroshima University, Hiroshima, Japan) for providing the RPA-expression plasmid and an E. coli strain to produce ss mp18 DNA, respectively. Several cloning vectors were obtained from the National BioResource Project (NIG, Japan). We thank Dr Haruo Ohmori (Institute for Virus Research, Kyoto University, Kyoto, Japan) and Dr Yoshihiro Matsumoto (Fox Chase Cancer Center, Philadelphia, USA) for helpful discussions. We are grateful to Eriko Aoki for her help with cDNA cloning, and Kumiko Mizuno, Tomoka Nakashima, Masako Okii, Hatsue Wakayama and Mai Yoshida for their laboratory assistance. This work was funded by Ministry of Education, Culture, Sports, Science and Technology of Japan (to Y.M., T.T., K.K.); the 21st Century Center of Excellence program from the Ministry of Education, Culture, Sports, Science and Technology of Japan (to K.K.); Research Fellowship for Young Scientists from Japan Society for the Promotion of Science (to J.P.); a scholarship award from the College Women's Association of Japan (to Y.G.). Funding to pay the Open Access publication charges for this article was provided by Grants-in-Aid from the Ministry of Education, Culture, Sports, Science and Technology of Japan (to K.K.).
Conflict of interest statement. None declared.
REFERENCES
- 1.Hübscher U, Maga G, Spadari S. Eukaryotic DNA polymerases. Annu. Rev. Biochem. 2002;71:133–163. doi: 10.1146/annurev.biochem.71.090501.150041. [DOI] [PubMed] [Google Scholar]
- 2.Johnson A, O’Donnell M. Cellular DNA replicases: components and dynamics at the replication fork. Annu. Rev. Biochem. 2005;74:283–315. doi: 10.1146/annurev.biochem.73.011303.073859. [DOI] [PubMed] [Google Scholar]
- 3.Waga S, Stillman B. The DNA replication fork in eukaryotic cells. Annu. Rev. Biochem. 1998;67:721–751. doi: 10.1146/annurev.biochem.67.1.721. [DOI] [PubMed] [Google Scholar]
- 4.Stillman B. Initiation of eukaryotic DNA replication in vitro. Annu. Rev. Cell. Biol. 1989;5:197–245. doi: 10.1146/annurev.cb.05.110189.001213. [DOI] [PubMed] [Google Scholar]
- 5.MacNeill SA, Baldacci G, Burgers PM, Hübscher U. A unified nomenclature for the subunits of eukaryotic DNA polymerase δ. Trends Biochem. Sci. 2001;26:16–17. doi: 10.1016/s0968-0004(00)01709-6. [DOI] [PubMed] [Google Scholar]
- 6.Lee SH, Kwong AD, Pan ZQ, Hurwitz J. Studies on the activator 1 protein complex, an accessory factor for proliferating cell nuclear antigen-dependent DNA polymerase δ. J. Biol. Chem. 1991;266:594–602. [PubMed] [Google Scholar]
- 7.Tsurimoto T, Stillman B. Purification of a cellular replication factor, RF-C, that is required for coordinated synthesis of leading and lagging strands during simian virus 40 DNA replication in vitro. Mol. Cell. Biol. 1989;9:609–619. doi: 10.1128/mcb.9.2.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tsurimoto T, Stillman B. Functions of replication factor C and proliferating-cell nuclear antigen: functional similarity of DNA polymerase accessory proteins from human cells and bacteriophage T4. Proc. Natl Acad. Sci. USA. 1990;87:1023–1027. doi: 10.1073/pnas.87.3.1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gulbis JM, Kelman Z, Hurwitz J, O’Donnell M, Kuriyan J. Structure of the C-terminal region of p21(WAF1/CIP1) complexed with human PCNA. Cell. 1996;87:297–306. doi: 10.1016/s0092-8674(00)81347-1. [DOI] [PubMed] [Google Scholar]
- 10.Krishna TS, Kong XP, Gary S, Burgers PM, Kuriyan J. Crystal structure of the eukaryotic DNA polymerase processivity factor PCNA. Cell. 1994;79:1233–1243. doi: 10.1016/0092-8674(94)90014-0. [DOI] [PubMed] [Google Scholar]
- 11.Bravo R, Frank R, Blundell PA, Macdonald-Bravo H. Cyclin/PCNA is the auxiliary protein of DNA polymerase-δ. Nature. 1987;326:515–517. doi: 10.1038/326515a0. [DOI] [PubMed] [Google Scholar]
- 12.Einolf HJ, Guengerich FP. Kinetic analysis of nucleotide incorporation by mammalian DNA polymerase δ. J. Biol. Chem. 2000;275:16316–16322. doi: 10.1074/jbc.M001291200. [DOI] [PubMed] [Google Scholar]
- 13.McConnell M, Miller H, Mozzherin DJ, Quamina A, Tan CK, Downey KM, Fisher PA. The mammalian DNA polymerase δ—proliferating cell nuclear antigen—template-primer complex: molecular characterization by direct binding. Biochemistry. 1996;35:8268–8274. doi: 10.1021/bi9530649. [DOI] [PubMed] [Google Scholar]
- 14.Ng L, McConnell M, Tan CK, Downey KM, Fisher PA. Interaction of DNA polymerase δ, proliferating cell nuclear antigen, and synthetic oligonucleotide template-primers. Analysis by polyacrylamide gel electrophoresis-band mobility shift assay. J. Biol. Chem. 1993;268:13571–13576. [PubMed] [Google Scholar]
- 15.Prelich G, Tan CK, Kostura M, Mathews MB, So AG, Downey KM, Stillman B. Functional identity of proliferating cell nuclear antigen and a DNA polymerase-δ auxiliary protein. Nature. 1987;326:517–520. doi: 10.1038/326517a0. [DOI] [PubMed] [Google Scholar]
- 16.Tan CK, Castillo C, So AG, Downey KM. An auxiliary protein for DNA polymerase-δ from fetal calf thymus. J. Biol. Chem. 1986;261:12310–12316. [PubMed] [Google Scholar]
- 17.Wold MS, Weinberg DH, Virshup DM, Li JJ, Kelly TJ. Identification of cellular proteins required for simian virus 40 DNA replication. J. Biol. Chem. 1989;264:2801–2809. [PubMed] [Google Scholar]
- 18.Wold MS. Replication protein A: a heterotrimeric, single-stranded DNA-binding protein required for eukaryotic DNA metabolism. Annu. Rev. Biochem. 1997;66:61–92. doi: 10.1146/annurev.biochem.66.1.61. [DOI] [PubMed] [Google Scholar]
- 19.Eki T, Matsumoto T, Murakami Y, Hurwitz J. The replication of DNA containing the simian virus 40 origin by the monopolymerase and dipolymerase systems. J. Biol. Chem. 1992;267:7284–7294. [PubMed] [Google Scholar]
- 20.Maga G, Stucki M, Spadari S, Hübscher U. DNA polymerase switching: I. replication factor C displaces DNA polymerase α prior to PCNA loading. J. Mol. Biol. 2000;295:791–801. doi: 10.1006/jmbi.1999.3394. [DOI] [PubMed] [Google Scholar]
- 21.Matsumoto T, Eki T, Hurwitz J. Studies on the initiation and elongation reactions in the simian virus 40 DNA replication system. Proc. Natl Acad. Sci. USA. 1990;87:9712–9716. doi: 10.1073/pnas.87.24.9712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mossi R, Keller RC, Ferrari E, Hübscher U. DNA polymerase switching: II. replication factor C abrogates primer synthesis by DNA polymerase α at a critical length. J. Mol. Biol. 2000;295:803–814. doi: 10.1006/jmbi.1999.3395. [DOI] [PubMed] [Google Scholar]
- 23.Tsurimoto T, Melendy T, Stillman B. Sequential initiation of lagging and leading strand synthesis by two different polymerase complexes at the SV40 DNA replication origin. Nature. 1990;346:534–539. doi: 10.1038/346534a0. [DOI] [PubMed] [Google Scholar]
- 24.Tsurimoto T, Stillman B. Replication factors required for SV40 DNA replication in vitro. II. switching of DNA polymerase α and δ during initiation of leading and lagging strand synthesis. J. Biol. Chem. 1991;266:1961–1968. [PubMed] [Google Scholar]
- 25.Waga S, Stillman B. Anatomy of a DNA replication fork revealed by reconstitution of SV40 DNA replication in vitro. Nature. 1994;369:207–212. doi: 10.1038/369207a0. [DOI] [PubMed] [Google Scholar]
- 26.Yuzhakov A, Kelman Z, Hurwitz J, O’Donnell M. Multiple competition reactions for RPA order the assembly of the DNA polymerase δ holoenzyme. EMBO J. 1999;18:6189–6199. doi: 10.1093/emboj/18.21.6189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Moldovan GL, Pfander B, Jentsch S. PCNA, the maestro of the replication fork. Cell. 2007;129:665–679. doi: 10.1016/j.cell.2007.05.003. [DOI] [PubMed] [Google Scholar]
- 28.Fukuda K, Morioka H, Imajou S, Ikeda S, Ohtsuka E, Tsurimoto T. Structure-function relationship of the eukaryotic DNA replication factor, proliferating cell nuclear antigen. J. Biol. Chem. 1995;270:22527–22534. doi: 10.1074/jbc.270.38.22527. [DOI] [PubMed] [Google Scholar]
- 29.Henricksen LA, Umbricht CB, Wold MS. Recombinant replication protein A: expression, complex formation, and functional characterization. J. Biol. Chem. 1994;269:11121–11132. [PubMed] [Google Scholar]
- 30.Burgers PM, Gerik KJ. Structure and processivity of two forms of Saccharomyces cerevisiae DNA polymerase δ. J. Biol. Chem. 1998;273:19756–19762. doi: 10.1074/jbc.273.31.19756. [DOI] [PubMed] [Google Scholar]
- 31.Sambrook J, Fritsch EF, Maniatis T. Molecular Cloning: A Laboratory Manual. 2nd. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory; 1989. [Google Scholar]
- 32.Wu CA, Zechner EL, Marians KJ. Coordinated leading- and lagging-strand synthesis at the Escherichia coli DNA replication fork. I. multiple effectors act to modulate Okazaki fragment size. J. Biol. Chem. 1992;267:4030–4044. [PubMed] [Google Scholar]
- 33.Melendy T, Stillman B. Purification of DNA polymerase δ as an essential simian virus 40 DNA replication factor. J. Biol. Chem. 1991;266:1942–1949. [PubMed] [Google Scholar]
- 34.Tsurimoto T, Stillman B. Multiple replication factors augment DNA synthesis by the two eukaryotic DNA polymerases, α and δ. EMBO J. 1989;8:3883–3889. doi: 10.1002/j.1460-2075.1989.tb08567.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Podust VN, Podust LM, Muller F, Hübscher U. DNA polymerase δ holoenzyme: action on single-stranded DNA and on double-stranded DNA in the presence of replicative DNA helicases. Biochemistry. 1995;34:5003–5010. doi: 10.1021/bi00015a011. [DOI] [PubMed] [Google Scholar]
- 36.Podust VN, Chang LS, Ott R, Dianov GL, Fanning E. Reconstitution of human DNA polymerase δ using recombinant baculoviruses: the p12 subunit potentiates DNA polymerizing activity of the four-subunit enzyme. J. Biol. Chem. 2002;277:3894–3901. doi: 10.1074/jbc.M109684200. [DOI] [PubMed] [Google Scholar]
- 37.Podust VN, Fanning E. Assembly of functional replication factor C expressed using recombinant baculoviruses. J. Biol. Chem. 1997;272:6303–6310. doi: 10.1074/jbc.272.10.6303. [DOI] [PubMed] [Google Scholar]
- 38.Podust VN, Tiwari N, Stephan S, Fanning E. Replication factor C disengages from proliferating cell nuclear antigen (PCNA) upon sliding clamp formation, and PCNA itself tethers DNA polymerase δ to DNA. J. Biol. Chem. 1998;273:31992–31999. doi: 10.1074/jbc.273.48.31992. [DOI] [PubMed] [Google Scholar]
- 39.Lee SH, Hurwitz J. Mechanism of elongation of primed DNA by DNA polymerase δ, proliferating cell nuclear antigen, and activator 1. Proc. Natl Acad. Sci. USA. 1990;87:5672–5676. doi: 10.1073/pnas.87.15.5672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tsurimoto T, Stillman B. Replication factors required for SV40 DNA replication in vitro. I. DNA structure-specific recognition of a primer-template junction by eukaryotic DNA polymerases and their accessory proteins. J. Biol. Chem. 1991;266:1950–1960. [PubMed] [Google Scholar]
- 41.Cai J, Uhlmann F, Gibbs E, Flores-Rozas H, Lee CG, Phillips B, Finkelstein J, Yao N, O’Donnell M, et al. Reconstitution of human replication factor C from its five subunits in baculovirus-infected insect cells. Proc. Natl Acad. Sci. USA. 1996;93:12896–12901. doi: 10.1073/pnas.93.23.12896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shibahara K, Stillman B. Replication-dependent marking of DNA by PCNA facilitates CAF-1-coupled inheritance of chromatin. Cell. 1999;96:575–585. doi: 10.1016/s0092-8674(00)80661-3. [DOI] [PubMed] [Google Scholar]
- 43.Yao N, Turner J, Kelman Z, Stukenberg PT, Dean F, Shechter D, Pan ZQ, Hurwitz J, O’Donnell M. Clamp loading, unloading and intrinsic stability of the PCNA, beta and gp45 sliding clamps of human, E. coli and T4 replicases. Genes Cells. 1996;1:101–113. doi: 10.1046/j.1365-2443.1996.07007.x. [DOI] [PubMed] [Google Scholar]
- 44.Burgers PM, Yoder BL. ATP-independent loading of the proliferating cell nuclear antigen requires DNA ends. J. Biol. Chem. 1993;268:19923–19926. [PubMed] [Google Scholar]
- 45.Zuo S, Bermudez V, Zhang G, Kelman Z, Hurwitz J. Structure and activity associated with multiple forms of Schizosaccharomyces pombe DNA polymerase δ. J. Biol. Chem. 2000;275:5153–5162. doi: 10.1074/jbc.275.7.5153. [DOI] [PubMed] [Google Scholar]
- 46.Li H, Xie B, Zhou Y, Rahmeh A, Trusa S, Zhang S, Gao Y, Lee EY, Lee MY. Functional roles of p12, the fourth subunit of human DNA polymerase δ. J. Biol. Chem. 2006;281:14748–14755. doi: 10.1074/jbc.M600322200. [DOI] [PubMed] [Google Scholar]
- 47.Gomes XV, Schmidt SL, Burgers PM. ATP utilization by yeast replication factor C. II. Multiple stepwise ATP binding events are required to load proliferating cell nuclear antigen onto primed DNA. J. Biol. Chem. 2001;276:34776–34783. doi: 10.1074/jbc.M011743200. [DOI] [PubMed] [Google Scholar]
- 48.Gomes XV, Burgers PM. ATP utilization by yeast replication factor C. I. ATP-mediated interaction with DNA and with proliferating cell nuclear antigen. J. Biol. Chem. 2001;276:34768–34775. doi: 10.1074/jbc.M011631200. [DOI] [PubMed] [Google Scholar]
- 49.Burgers PM. Saccharomyces cerevisiae replication factor C. II. Formation and activity of complexes with the proliferating cell nuclear antigen and with DNA polymerases δ and ε. J. Biol. Chem. 1991;266:22698–22706. [PubMed] [Google Scholar]
- 50.Walther AP, Bjerke MP, Wold MS. A novel assay for examining the molecular reactions at the eukaryotic replication fork: activities of replication protein A required during elongation. Nucleic Acids Res. 1999;27:656–664. doi: 10.1093/nar/27.2.656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Podust LM, Podust VN, Floth C, Hübscher U. Assembly of DNA polymerase δ and ε holoenzymes depends on the geometry of the DNA template. Nucleic Acids Res. 1994;22:2970–2975. doi: 10.1093/nar/22.15.2970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Waga S, Stillman B. Cyclin-dependent kinase inhibitor p21 modulates the DNA primer-template recognition complex. Mol. Cell. Biol. 1998;18:4177–4187. doi: 10.1128/mcb.18.7.4177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bylund GO, Burgers PM. Replication protein A-directed unloading of PCNA by the Ctf18 cohesion establishment complex. Mol. Cell. Biol. 2005;25:5445–5455. doi: 10.1128/MCB.25.13.5445-5455.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bermudez VP, MacNeill SA, Tappin I, Hurwitz J. The influence of the Cdc27 subunit on the properties of the Schizosaccharomyces pombe DNA polymerase δ. J. Biol. Chem. 2002;277:36853–36862. doi: 10.1074/jbc.M202897200. [DOI] [PubMed] [Google Scholar]
- 55.Ducoux M, Urbach S, Baldacci G, Hübscher U, Koundrioukoff S, Christensen J, Hughes P. Mediation of proliferating cell nuclear antigen (PCNA)-dependent DNA replication through a conserved p21(Cip1)-like PCNA-binding motif present in the third subunit of human DNA polymerase δ. J. Biol. Chem. 2001;276:49258–49266. doi: 10.1074/jbc.M106990200. [DOI] [PubMed] [Google Scholar]
- 56.Johansson E, Garg P, Burgers PM. The Pol32 subunit of DNA polymerase δ contains separable domains for processive replication and proliferating cell nuclear antigen (PCNA) binding. J. Biol. Chem. 2004;279:1907–1915. doi: 10.1074/jbc.M310362200. [DOI] [PubMed] [Google Scholar]
- 57.Reynolds N, Warbrick E, Fantes PA, MacNeill SA. Essential interaction between the fission yeast DNA polymerase δ subunit Cdc27 and Pcn1 (PCNA) mediated through a C-terminal p21(Cip1)-like PCNA binding motif. EMBO J. 2000;19:1108–1118. doi: 10.1093/emboj/19.5.1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Podust LM, Podust VN, Sogo JM, Hübscher U. Mammalian DNA polymerase auxiliary proteins: analysis of replication factor C-catalyzed proliferating cell nuclear antigen loading onto circular double-stranded DNA. Mol. Cell. Biol. 1995;15:3072–3081. doi: 10.1128/mcb.15.6.3072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pursell ZF, Isoz I, Lundstrom EB, Johansson E, Kunkel TA. Yeast DNA polymerase ε participates in leading-strand DNA replication. Science. 2007;317:127–130. doi: 10.1126/science.1144067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Shcherbakova PV, Pavlov YI. 3′ → 5′ exonuclease of DNA polymerase ε and δ correct base analog induced DNA replication errors on opposite DNA strands in Saccharomyces cerevisiae. Genetics. 1996;142:717–726. doi: 10.1093/genetics/142.3.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Levin DS, Vijayakumar S, Liu X, Bermudez VP, Hurwitz J, Tomkinson AE. A conserved interaction between the replicative clamp loader and DNA ligase in eukaryotes: implications for Okazaki fragment joining. J. Biol. Chem. 2004;279:55196–55201. doi: 10.1074/jbc.M409250200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.