Abstract
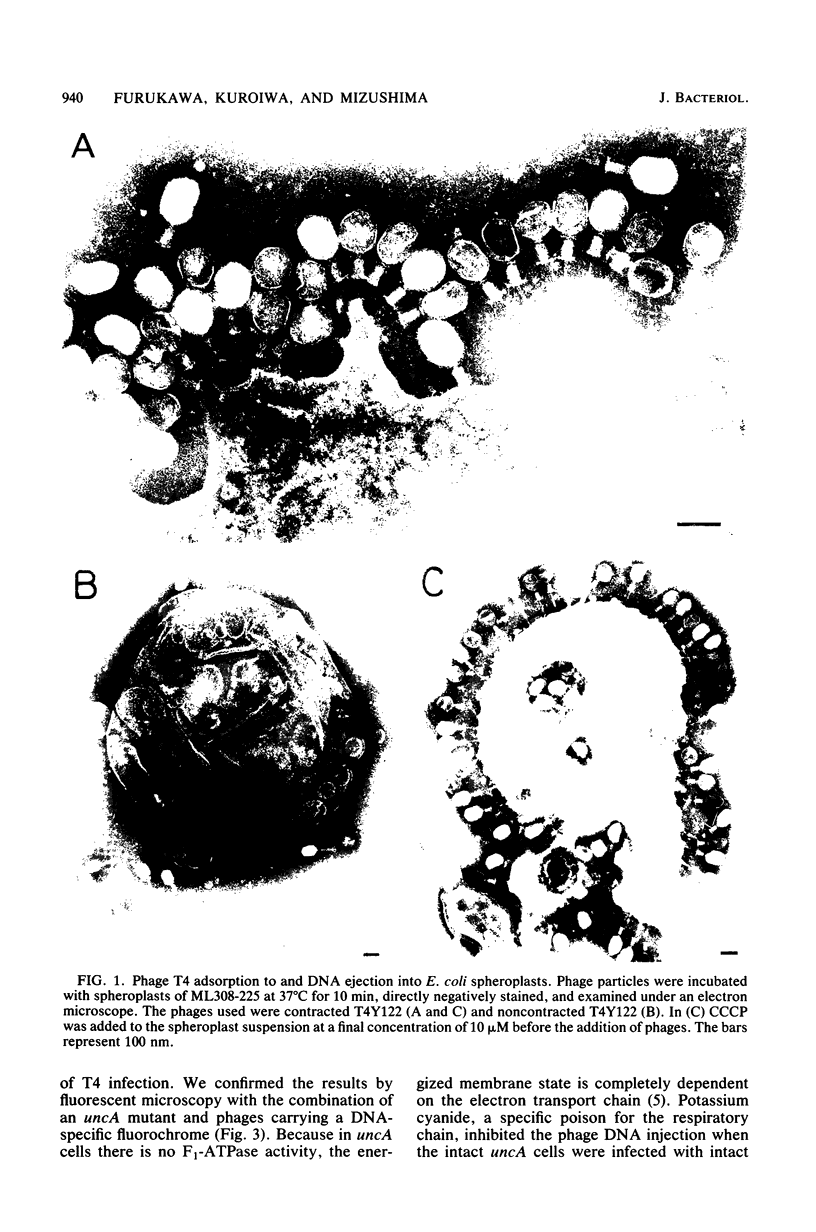
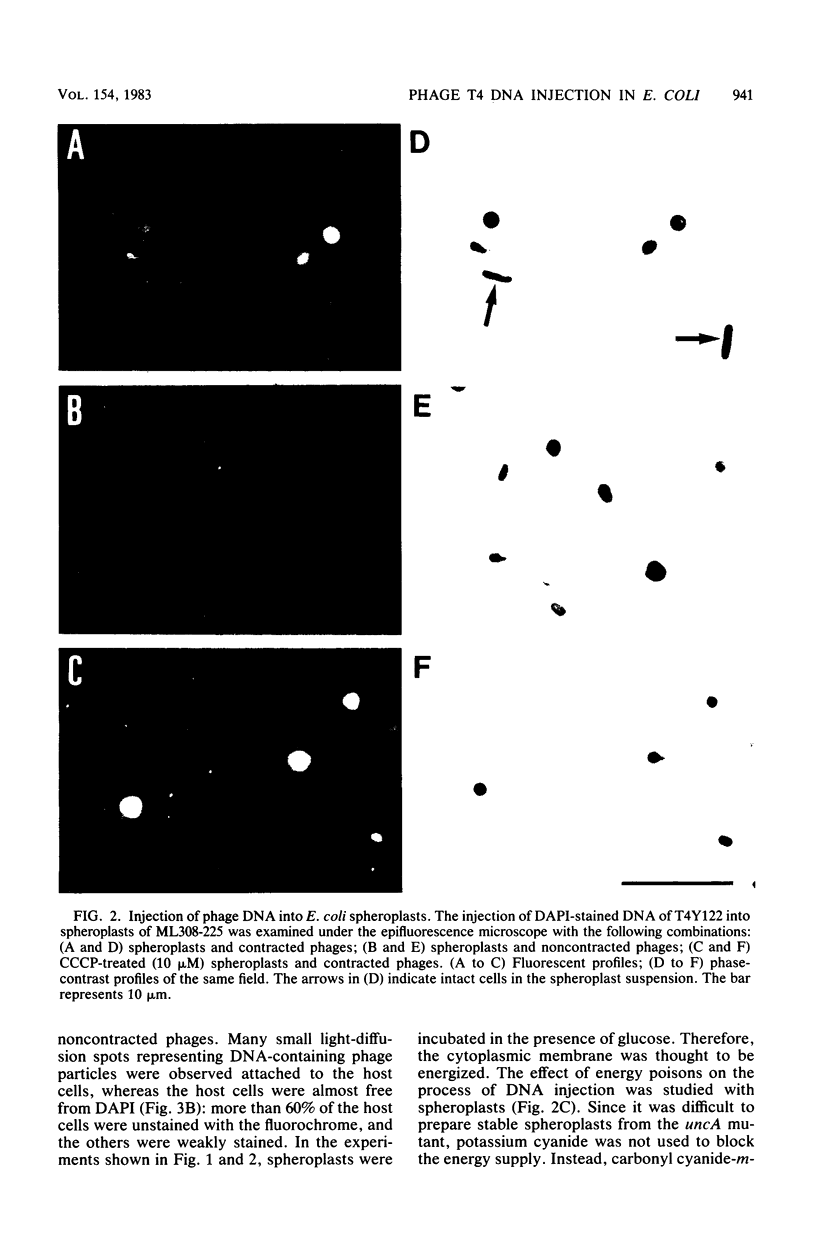
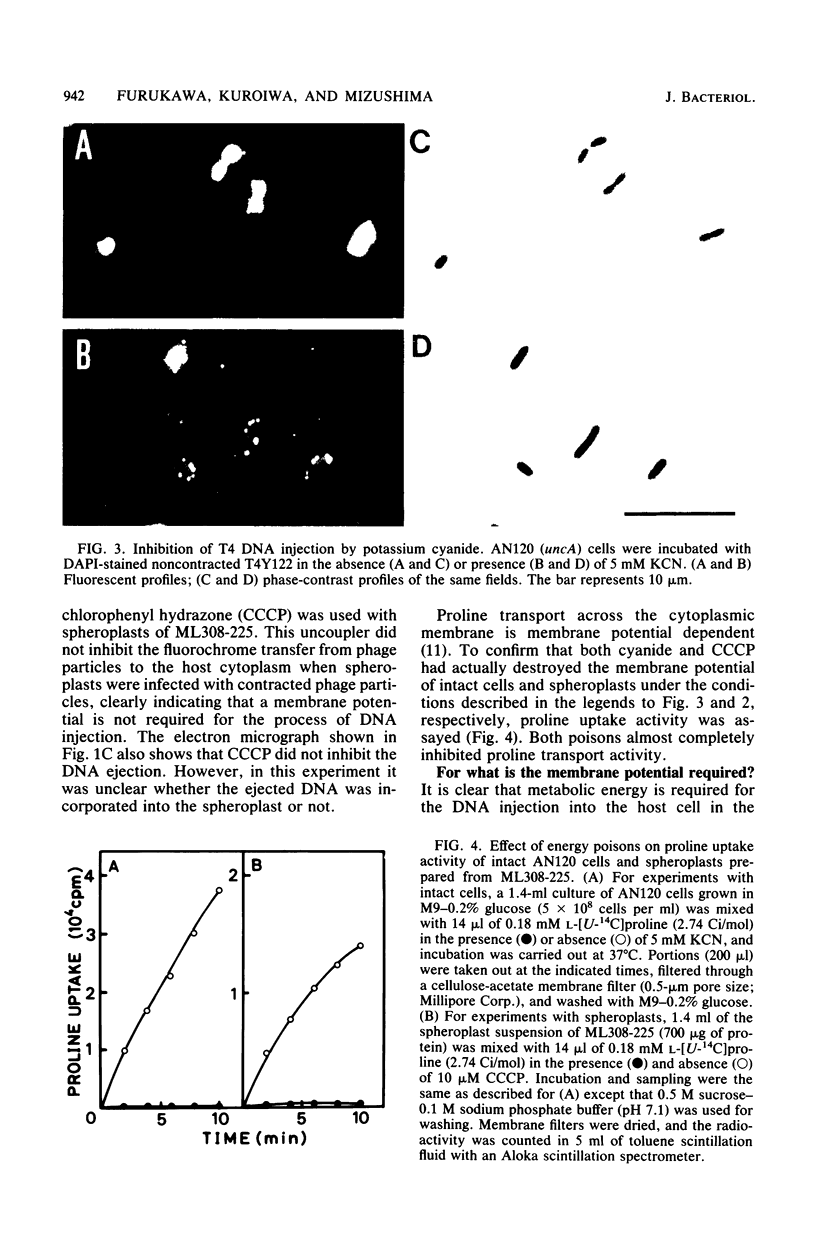
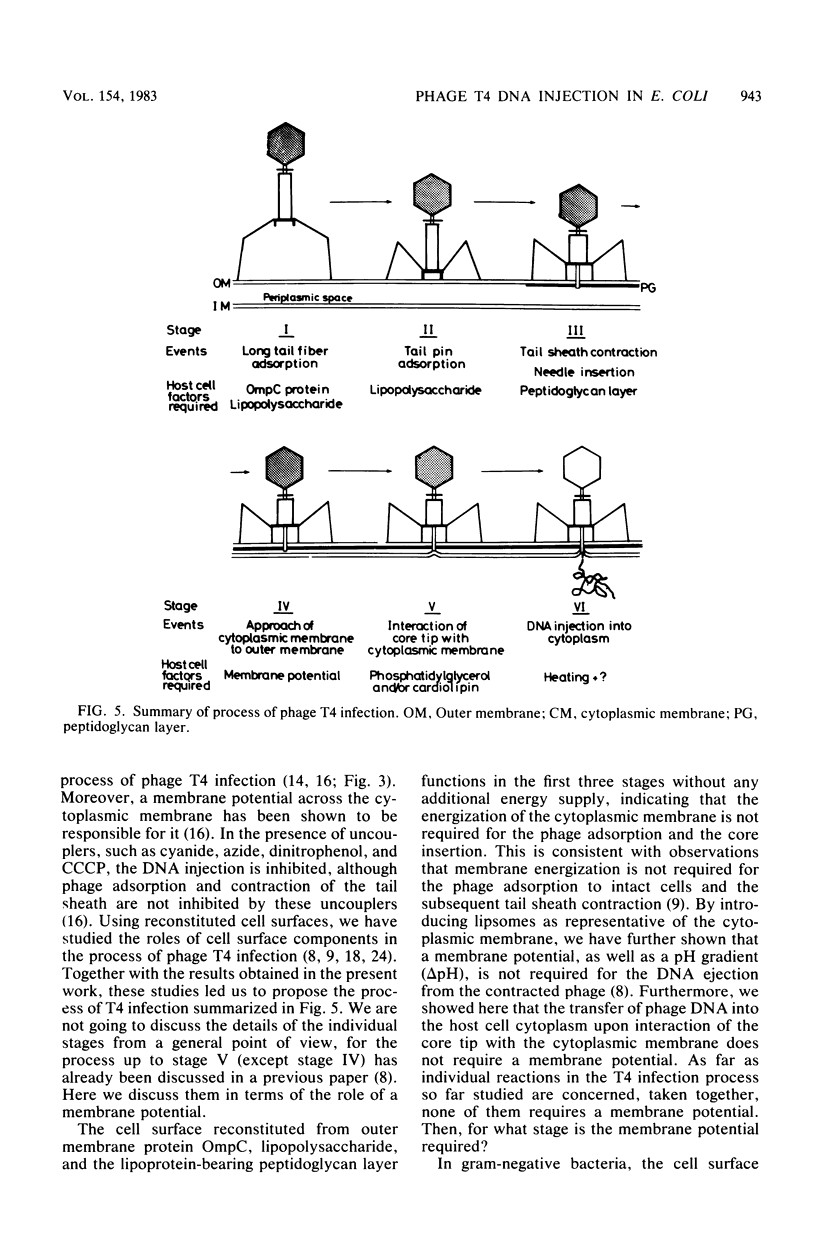
The process of phage T4 DNA injection into the host cell was studied under a fluorescent microscope, using 4',6-diamidino-2-phenylindole as a DNA-specific fluorochrome. The phage DNA injection was observed when spheroplasts were infected with the artificially contracted phage particles having a protruding core. The DNA injection was mediated by the interaction of the core tip with the cytoplasmic membrane of the spheroplast. A membrane potential was not required for the process of DNA injection. On the other hand, DNA injection upon infection by intact noncontracted phage of the intact host cell was inhibited by an energy poison. Based on these observations, together with results from previous work, a model for the T4 infection process is presented, and the role of the membrane potential in the infection process is discussed.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bayer M. E. Adsorption of bacteriophages to adhesions between wall and membrane of Escherichia coli. J Virol. 1968 Apr;2(4):346–356. doi: 10.1128/jvi.2.4.346-356.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayer M. E. Areas of adhesion between wall and membrane of Escherichia coli. J Gen Microbiol. 1968 Oct;53(3):395–404. doi: 10.1099/00221287-53-3-395. [DOI] [PubMed] [Google Scholar]
- Bayer M. E., Bayer M. H. Fast responses of bacterial membranes to virus adsorption: a fluorescence study. Proc Natl Acad Sci U S A. 1981 Sep;78(9):5618–5622. doi: 10.1073/pnas.78.9.5618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayer M. E., Starkey T. W. The adsorption of bacteriophage phi X174 and its interaction with Escherichia coli; a kinetic and morphological study. Virology. 1972 Jul;49(1):236–256. doi: 10.1016/s0042-6822(72)80026-6. [DOI] [PubMed] [Google Scholar]
- Butlin J. D., Cox G. B., Gibson F. Oxidative phosphorylation in Escherichia coli K12. Mutations affecting magnesium ion- or calcium ion-stimulated adenosine triphosphatase. Biochem J. 1971 Aug;124(1):75–81. doi: 10.1042/bj1240075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donohue-Rolfe A. M., Schaechter M. Translocation of phospholipids from the inner to the outer membrane of Escherichia coli. Proc Natl Acad Sci U S A. 1980 Apr;77(4):1867–1871. doi: 10.1073/pnas.77.4.1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furukawa H., Mizushima S. Roles of cell surface components of Escherichia coli K-12 in bacteriophage T4 infection: interaction of tail core with phospholipids. J Bacteriol. 1982 May;150(2):916–924. doi: 10.1128/jb.150.2.916-924.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furukawa H., Yamada H., Mizushima S. Interaction of bacteriophage T4 with reconstituted cell envelopes of Escherichia coli K-12. J Bacteriol. 1979 Dec;140(3):1071–1080. doi: 10.1128/jb.140.3.1071-1080.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henning U., Jann K. Two-component nature of bacteriophage T4 receptor activity in Escherichia coli K-12. J Bacteriol. 1979 Jan;137(1):664–666. doi: 10.1128/jb.137.1.664-666.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirata H., Altendorf K., Harold F. M. Role of an electrical potential in the coupling of metabolic energy to active transport by membrane vesicles of Escherichia coli. Proc Natl Acad Sci U S A. 1973 Jun;70(6):1804–1808. doi: 10.1073/pnas.70.6.1804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jetten A. M., Jetten M. E. Energy requirement for the initiation of colicin action in Escherichia coli. Biochim Biophys Acta. 1975 Apr 14;387(1):12–22. doi: 10.1016/0005-2728(75)90048-1. [DOI] [PubMed] [Google Scholar]
- Kalasauskaite E., Grinius L. The role of energy-yielding ATPase and respiratory chain at early stages of bacteriophage T4 infection. FEBS Lett. 1979 Mar 15;99(2):287–291. doi: 10.1016/0014-5793(79)80974-6. [DOI] [PubMed] [Google Scholar]
- Konisky J., Tokuda H. Mode of action of colicins Ia, E1 and K. Zentralbl Bakteriol Orig A. 1979 Jun;244(1):105–120. [PubMed] [Google Scholar]
- Labedan B., Goldberg E. B. Requirement for membrane potential in injection of phage T4 DNA. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4669–4673. doi: 10.1073/pnas.76.9.4669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizushima S., Yamada H. Isolation and characterization of two outer membrane preparations from Escherichia coli. Biochim Biophys Acta. 1975 Jan 14;375(1):44–53. doi: 10.1016/0005-2736(75)90071-1. [DOI] [PubMed] [Google Scholar]
- Mutoh N., Furukawa H., Mizushima S. Role of lipopolysaccharide and outer membrane protein of Escherichia coli K-12 in the receptor activity for bacteriophage T4. J Bacteriol. 1978 Nov;136(2):693–699. doi: 10.1128/jb.136.2.693-699.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieva-Gomez D., Konisky J. Membrane changes in Escherichia coli induced by colicin Ia and agents known to disrupt energy transduction. Biochemistry. 1976 Jun 29;15(13):2747–2753. doi: 10.1021/bi00658a006. [DOI] [PubMed] [Google Scholar]
- Okamoto K. Requirement of heat and metabolic energy for the expression of inhibitory action of colicin K. Biochim Biophys Acta. 1975 May 6;389(2):370–379. doi: 10.1016/0005-2736(75)90329-6. [DOI] [PubMed] [Google Scholar]
- Schleyer M., Schmidt B., Neupert W. Requirement of a membrane potential for the posttranslational transfer of proteins into mitochondria. Eur J Biochem. 1982 Jun 15;125(1):109–116. doi: 10.1111/j.1432-1033.1982.tb06657.x. [DOI] [PubMed] [Google Scholar]
- Szoka F., Jr, Papahadjopoulos D. Procedure for preparation of liposomes with large internal aqueous space and high capture by reverse-phase evaporation. Proc Natl Acad Sci U S A. 1978 Sep;75(9):4194–4198. doi: 10.1073/pnas.75.9.4194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson D. H., Fennell D. J. The use of fluorescent DNA-binding agent for detecting and separating yeast mitochondrial DNA. Methods Cell Biol. 1975;12:335–351. doi: 10.1016/s0091-679x(08)60963-2. [DOI] [PubMed] [Google Scholar]
- Yamamoto M., Uchida H. Organization and function of bacteriophage T4 tail. I. Isolation of heat-sensitive T4 tail mutants. Virology. 1973 Mar;52(1):234–245. doi: 10.1016/0042-6822(73)90412-1. [DOI] [PubMed] [Google Scholar]
- Yu F., Mizushima S. Roles of lipopolysaccharide and outer membrane protein OmpC of Escherichia coli K-12 in the receptor function for bacteriophage T4. J Bacteriol. 1982 Aug;151(2):718–722. doi: 10.1128/jb.151.2.718-722.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]