Abstract
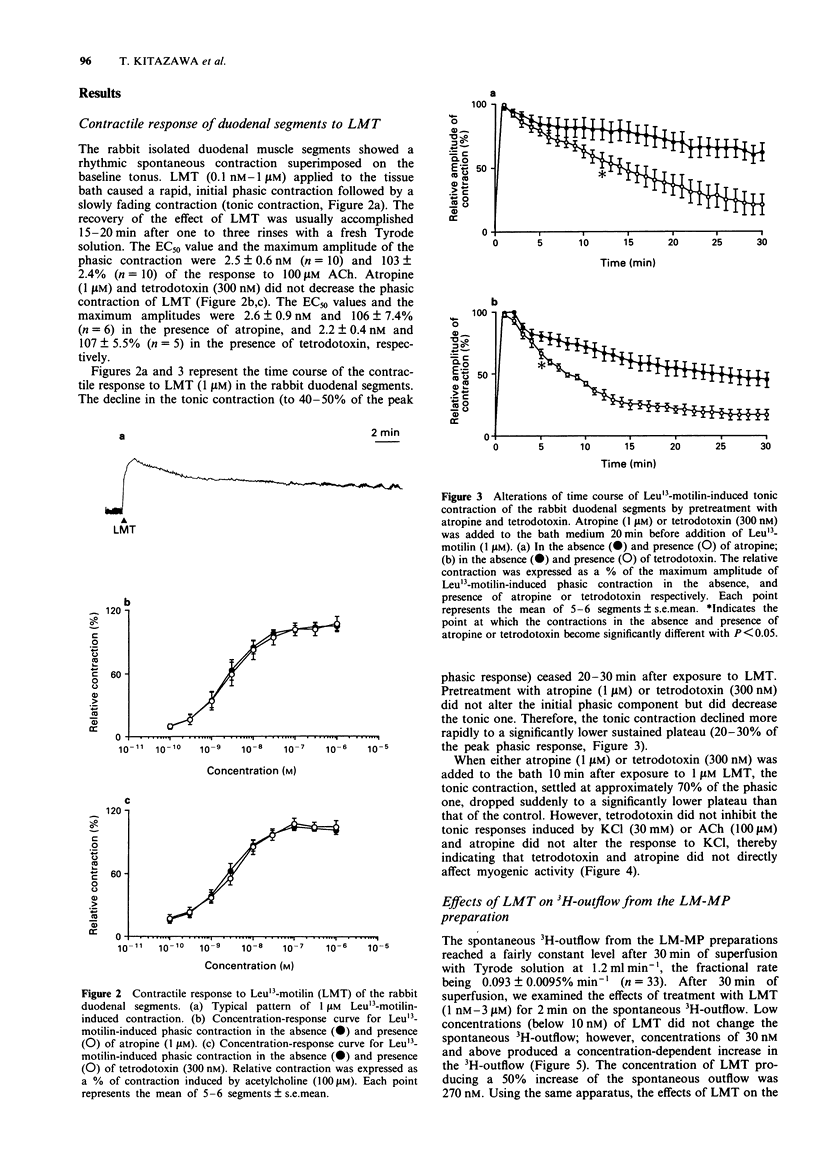
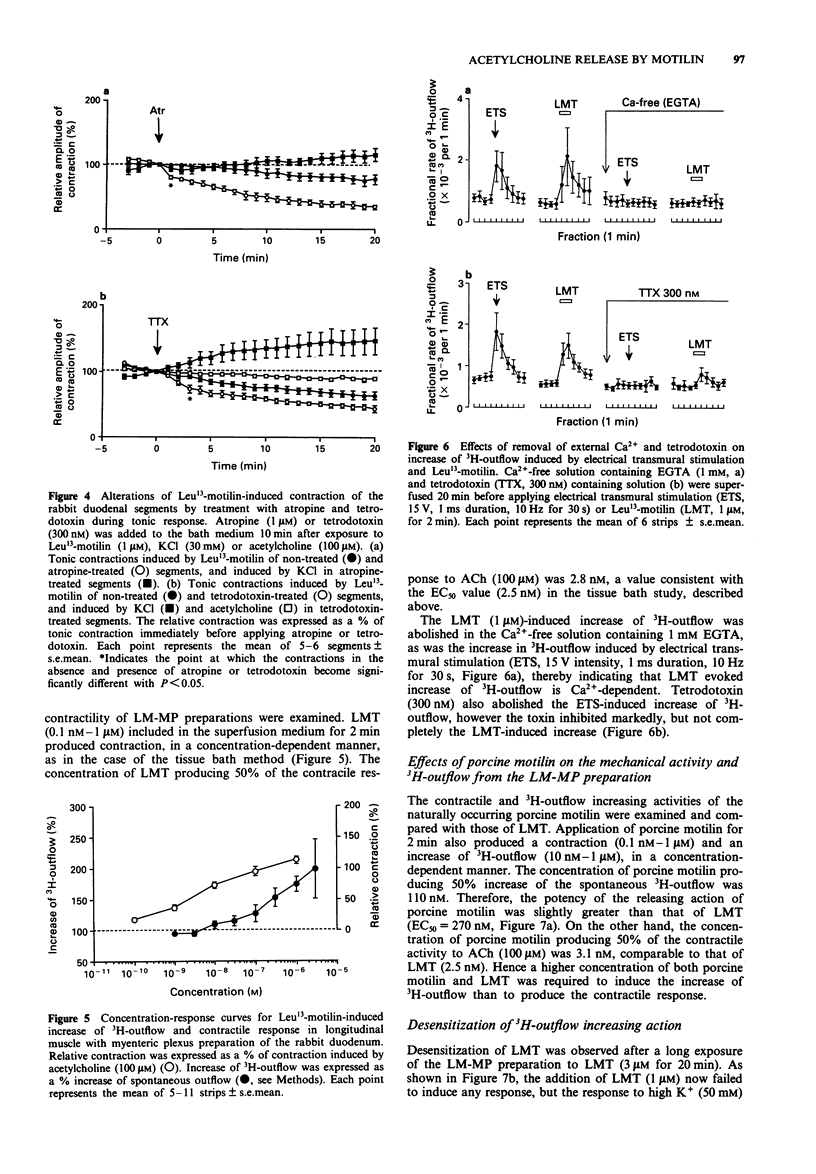
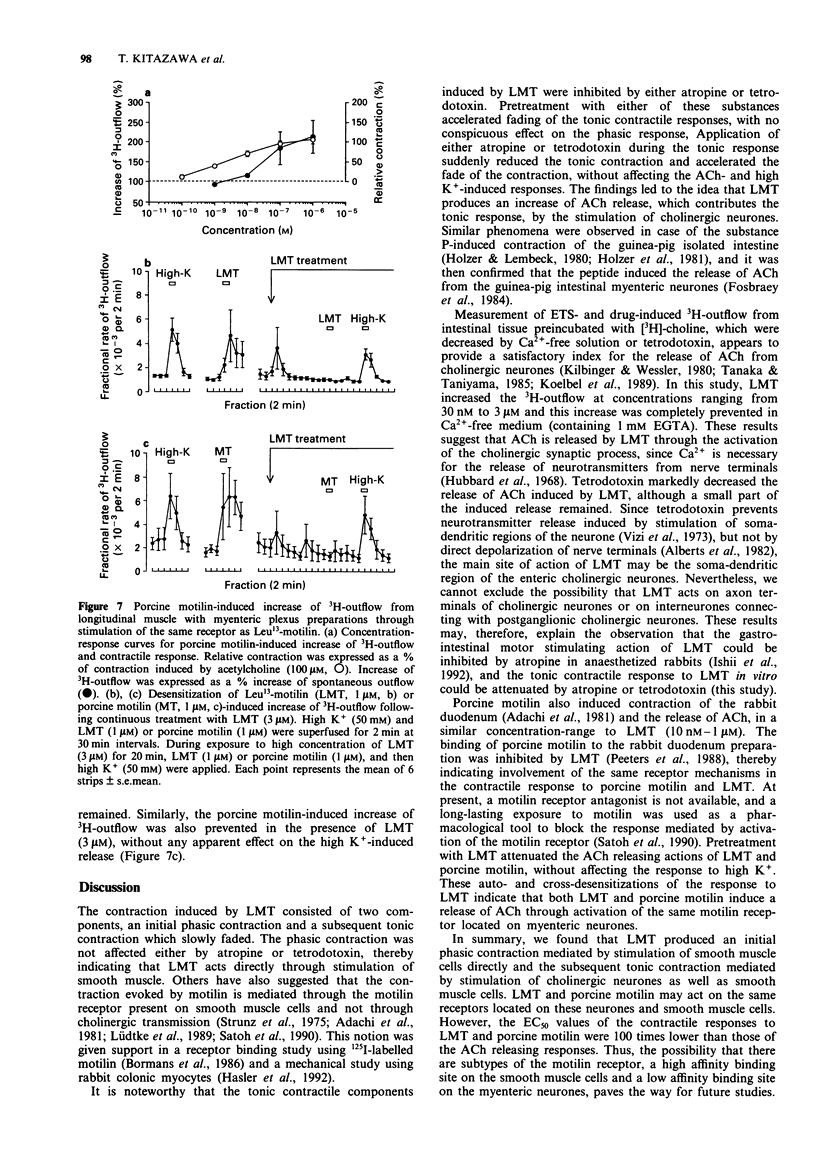
1. Involvement of cholinergic mechanisms in the contractile response to Leu13-motilin (LMT, KW-5139) was investigated in rabbit duodenal segments, and longitudinal muscle-myenteric plexus (LM-MP) preparations preincubated wtih [3H]-choline. 2. Contractile response to LMT (0.1 nM-1 microM) consisted of an initial rapid (phasic) contraction and a tonic contraction slowly fading to a sustained plateau. LMT caused a concentration-dependent phasic contraction of rabbit isolated duodenal segments. The EC50 value was 2.5 nM and the maximum amplitude of the contraction was 103% of the response induced by acetylcholine (ACh, 100 microM). Neither tetrodotoxin nor atropine changed the EC50 value or the maximum amplitude of the response to LMT. 3. Both atropine and tetrodotoxin decreased the amplitude and accelerated fading of the tonic contraction produced by LMT. 4. LMT (30 nM-3 microM) induced an increase of 3H-outflow, in a concentration-dependent manner. The LMT-induced increase of 3H-outflow was prevented by removal of external Ca2+ or by the presence of tetrodotoxin. 5. Porcine motilin (10 nM-1 microM) also stimulated the release of 3H at a similar concentration-range to that seen with LMT. 6. Pretreatment with LMT (3 microM for 20 min) decreased LMT- and the porcine motilin-evoked release of 3H but did not alter the high K(+)-evoked release. 7. Our results suggest that LMT and porcine motilin stimulate the release of ACh from enteric neurones through the same receptor, and that the release of ACh plays a role in tonic components of contraction in the rabbit duodenum.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AMBACHE N. Separation of the longitudinal muscle of the rabbit's ileum as a broad sheet. J Physiol. 1954 Aug 27;125(2):53–5P. [PubMed] [Google Scholar]
- Adachi H., Toda N., Hayashi S., Noguchi M., Suzuki T., Torizuka K., Yajima H., Koyama K. Mechanism of the excitatory action of motilin on isolated rabbit intestine. Gastroenterology. 1981 Apr;80(4):783–788. [PubMed] [Google Scholar]
- Alberts P., Bartfai T., Stjärne L. The effects of atropine on [3H]acetylcholine secretion from guinea-pig myenteric plexus evoked electrically or by high potassium. J Physiol. 1982 Aug;329:93–112. doi: 10.1113/jphysiol.1982.sp014292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bormans V., Peeters T. L., Vantrappen G. Motilin receptors in rabbit stomach and small intestine. Regul Pept. 1986 Sep;15(2):143–153. doi: 10.1016/0167-0115(86)90084-4. [DOI] [PubMed] [Google Scholar]
- Brown J. C., Cook M. A., Dryburgh J. R. Motilin, a gastric motor activity stimulating polypeptide: the complete amino acid sequence. Can J Biochem. 1973 May;51(5):533–537. doi: 10.1139/o73-066. [DOI] [PubMed] [Google Scholar]
- Brown J. C., Mutt V., Dryburgh J. R. The further purification of motilin, a gastric motor activity stimulating polypeptide from the mucosa of the small intestine of hogs. Can J Physiol Pharmacol. 1971 May;49(5):399–405. doi: 10.1139/y71-047. [DOI] [PubMed] [Google Scholar]
- Fosbraey P., Featherstone R. L., Morton I. K. Comparison of potency of substance P and related peptides on [3H]-acetylcholine release, and contractile actions, in the guinea-pig ileum. Naunyn Schmiedebergs Arch Pharmacol. 1984 Jun;326(2):111–115. doi: 10.1007/BF00517306. [DOI] [PubMed] [Google Scholar]
- Fox J. E., Daniel E. E., Jury J., Robotham H. The mechanism of motilin excitation of the canine small intestine. Life Sci. 1984 Mar 5;34(10):1001–1006. doi: 10.1016/0024-3205(84)90305-9. [DOI] [PubMed] [Google Scholar]
- Hasler W. L., Heldsinger A., Chung O. Y. Erythromycin contracts rabbit colon myocytes via occupation of motilin receptors. Am J Physiol. 1992 Jan;262(1 Pt 1):G50–G55. doi: 10.1152/ajpgi.1992.262.1.G50. [DOI] [PubMed] [Google Scholar]
- Helmstaedter V., Kreppein W., Domschke W., Mitznegg P., Yanaihara N., Wünsch E., Forssmann W. G. Immunohistochemical localization of motilin in endocrine non-enterochromaffin cells of the small intestine of humans and monkey. Gastroenterology. 1979 May;76(5 Pt 1):897–902. [PubMed] [Google Scholar]
- Hirning L. D., Burks T. F. Neurogenic mechanism of action of motilin in the canine isolated small intestine ex vivo. Eur J Pharmacol. 1986 Sep 9;128(3):241–248. doi: 10.1016/0014-2999(86)90771-5. [DOI] [PubMed] [Google Scholar]
- Holzer P., Emson P. C., Iversen L. L., Sharman D. F. Regional differences in the response to substance P on the longitudinal muscle and the concentration of substance P in the digestive tract of the guinea-pig. Neuroscience. 1981;6(7):1433–1441. doi: 10.1016/0306-4522(81)90198-6. [DOI] [PubMed] [Google Scholar]
- Holzer P., Lembeck F. Neurally mediated contraction of ileal longitudinal muscle by substance P. Neurosci Lett. 1980 Apr;17(1-2):101–105. doi: 10.1016/0304-3940(80)90069-5. [DOI] [PubMed] [Google Scholar]
- Hubbard J. I., Jones S. F., Landau E. M. On the mechanism by which calcium and magnesium affect the release of transmitter by nerve impulses. J Physiol. 1968 May;196(1):75–86. doi: 10.1113/jphysiol.1968.sp008495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inatomi N., Satoh H., Maki Y., Hashimoto N., Itoh Z., Omura S. An erythromycin derivative, EM-523, induces motilin-like gastrointestinal motility in dogs. J Pharmacol Exp Ther. 1989 Nov;251(2):707–712. [PubMed] [Google Scholar]
- Itoh Z., Honda R., Hiwatashi K., Takeuchi S., Aizawa I., Takayanagi R., Couch E. F. Motilin-induced mechanical activity in the canine alimentary tract. Scand J Gastroenterol Suppl. 1976;39:93–110. [PubMed] [Google Scholar]
- Janssens J., Vantrappen G., Peeters T. L. The activity front of the migrating motor complex of the human stomach but not of the small intestine is motilin-dependent. Regul Pept. 1983 Aug;6(4):363–369. doi: 10.1016/0167-0115(83)90265-3. [DOI] [PubMed] [Google Scholar]
- Kilbinger H., Wessler I. Inhibition by acetylcholine of the stimulation-evoked release of [3H]acetylcholine from the guinea-pig myenteric plexus. Neuroscience. 1980;5(7):1331–1340. doi: 10.1016/0306-4522(80)90205-5. [DOI] [PubMed] [Google Scholar]
- Koelbel C. B., Mayer E. A., Reeve J. R., Jr, Snape W. J., Jr, Patel A., Ho F. J. Involvement of substance P in noncholinergic excitation of rabbit colonic muscle. Am J Physiol. 1989 Jan;256(1 Pt 1):G246–G253. doi: 10.1152/ajpgi.1989.256.1.G246. [DOI] [PubMed] [Google Scholar]
- Lüdtke F. E., Müller H., Golenhofen K. Direct effects of motilin on isolated smooth muscle from various regions of the human stomach. Pflugers Arch. 1989 Sep;414(5):558–563. doi: 10.1007/BF00580991. [DOI] [PubMed] [Google Scholar]
- Satoh T., Inatomi N., Satoh H., Marui S., Itoh Z., Omura S. EM-523, an erythromycin derivative, and motilin show similar contractile activity in isolated rabbit intestine. J Pharmacol Exp Ther. 1990 Sep;254(3):940–944. [PubMed] [Google Scholar]
- Strunz U., Domschke W., Mitznegg P., Domschke S., Schubert E., Wünsch E., Jaeger E., Demling L. Analysis of the motor effects of 13-norleucine motilin on the rabbit, guinea pig, rat, and human alimentary tract in vitro. Gastroenterology. 1975 Jun;68(6):1485–1491. [PubMed] [Google Scholar]
- Tanaka C., Taniyama K. Substance P provoked gamma-aminobutyric acid release from the myenteric plexus of the guinea-pig small intestine. J Physiol. 1985 May;362:319–329. doi: 10.1113/jphysiol.1985.sp015680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vizi S. E., Bertaccini G., Impicciatore M., Knoll J. Evidence that acetylcholine released by gastrin and related polypeptides contributes to their effect on gastrointestinal motility. Gastroenterology. 1973 Feb;64(2):268–277. [PubMed] [Google Scholar]


