Abstract
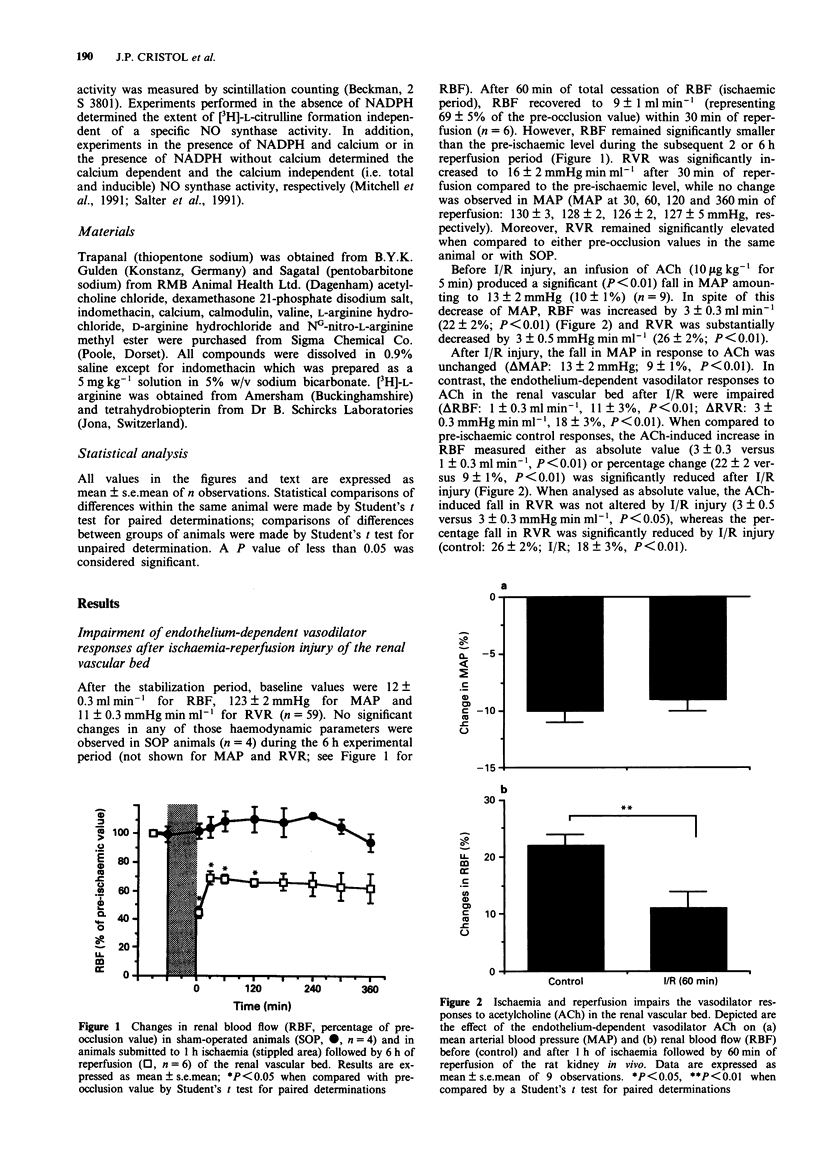
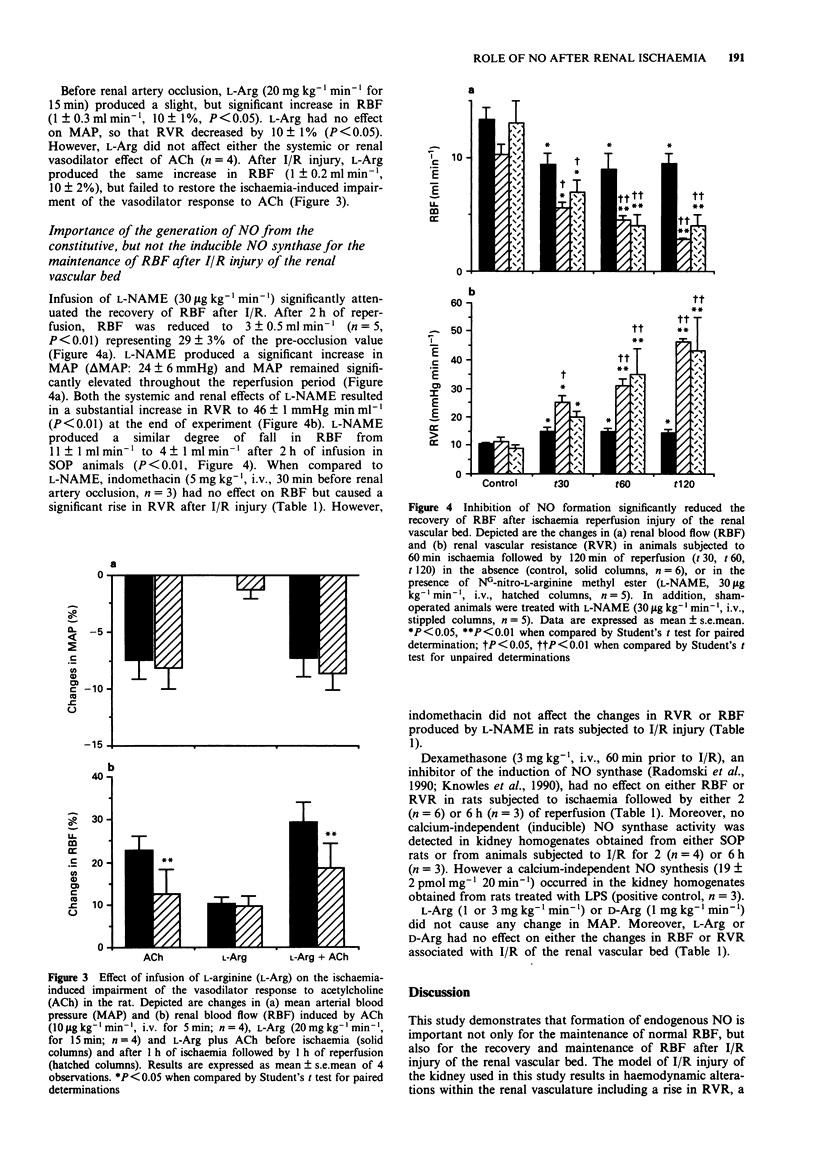
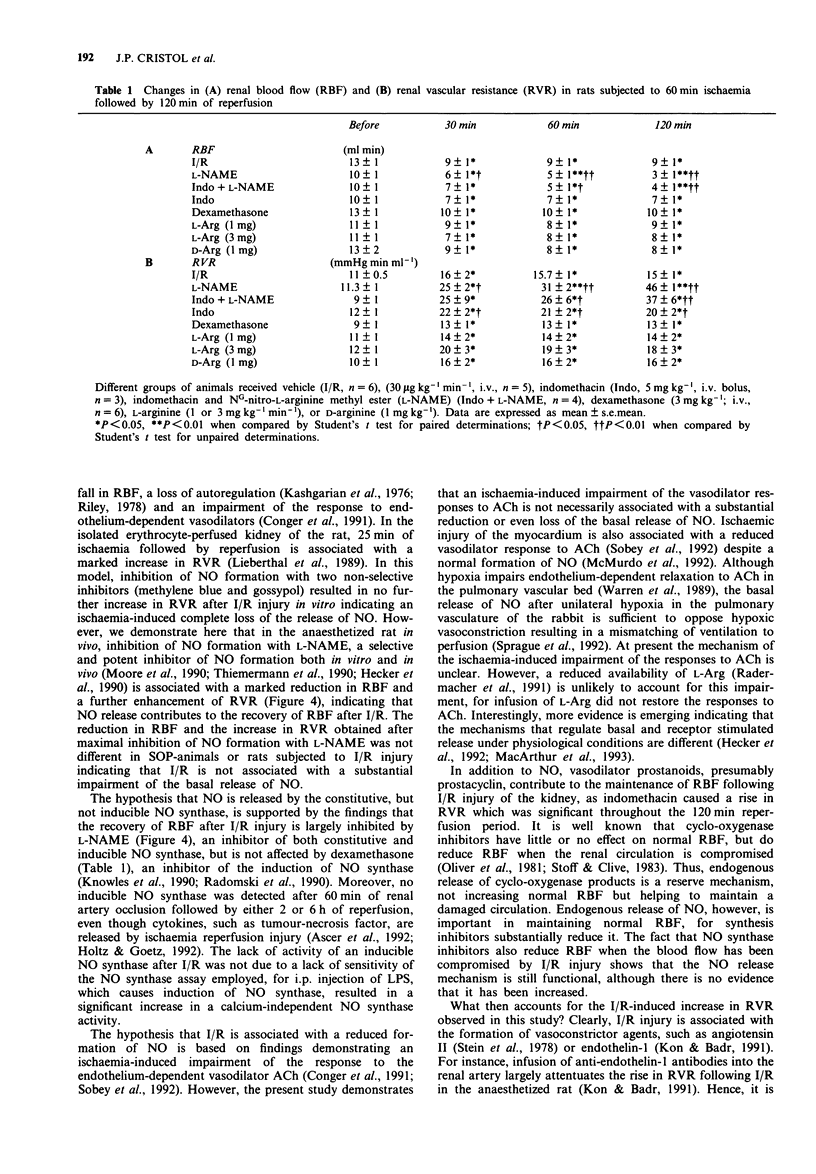
1. Ischaemia-reperfusion injury in the kidney is associated with a loss of autoregulation, an increase in renal vascular resistance (RVR), a decrease of renal blood flow (RBF) and ultimately acute renal failure. The aim of this study was to investigate the role of the release of endogenous nitric oxide (NO) in the recovery of RBF after ischaemic injury of the renal vascular bed. 2. Anaesthetized rats (thiopentone sodium; 120 mg kg-1, i.p.) were submitted to acute renal ischaemia followed by 2 or 6 h of reperfusion (I/R). Reperfusion was associated with a significant reduction in RBF, an increase in RVR, and an impairment of the vasodilator effect of acetylcholine (ACh). 3. NG-nitro-L-arginine methyl ester (L-NAME, 30 micrograms kg-1 min-1, i.v., n = 5) significantly prevented the recovery of RBF after I/R injury. Similarly, inhibition of prostanoid formation with indomethacin (5 mg kg-1, i.v., n = 4) significantly enhanced the rise in RVR associated with I/R injury. 4. Infusion of L-arginine (L-Arg; 1 or 3 mg kg-1 min-1, i.v., n = 5 and 4, respectively) or D-Arg (1 mg kg-1 min-1, i.v., n = 6), starting 30 min after occlusion, did not improve the recovery of RBF. Furthermore, infusion of L-Arg (20 mg kg-1 min-1 for 15 min; n = 4) had no effect on the I/R-induced impairment of the vasodilator responses to ACh.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ascer E., Mohan C., Gennaro M., Cupo S. Interleukin-1 and thromboxane release after skeletal muscle ischemia and reperfusion. Ann Vasc Surg. 1992 Jan;6(1):69–73. doi: 10.1007/BF02000671. [DOI] [PubMed] [Google Scholar]
- Baylis C., Harton P., Engels K. Endothelial derived relaxing factor controls renal hemodynamics in the normal rat kidney. J Am Soc Nephrol. 1990 Dec;1(6):875–881. doi: 10.1681/ASN.V16875. [DOI] [PubMed] [Google Scholar]
- Bhardwaj R., Moore P. K. The effect of arginine and nitric oxide on resistance blood vessels of the perfused rat kidney. Br J Pharmacol. 1989 Jul;97(3):739–744. doi: 10.1111/j.1476-5381.1989.tb12011.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bredt D. S., Snyder S. H. Isolation of nitric oxide synthetase, a calmodulin-requiring enzyme. Proc Natl Acad Sci U S A. 1990 Jan;87(2):682–685. doi: 10.1073/pnas.87.2.682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brezis M., Heyman S. N., Dinour D., Epstein F. H., Rosen S. Role of nitric oxide in renal medullary oxygenation. Studies in isolated and intact rat kidneys. J Clin Invest. 1991 Aug;88(2):390–395. doi: 10.1172/JCI115316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton G. A., MacNeil S., de Jonge A., Haylor J. Cyclic GMP release and vasodilatation induced by EDRF and atrial natriuretic factor in the isolated perfused kidney of the rat. Br J Pharmacol. 1990 Feb;99(2):364–368. doi: 10.1111/j.1476-5381.1990.tb14709.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chevalier R. L., Fern R. J., Garmey M., el-Dahr S. S., Gomez R. A., De Vente J. Localization of cGMP after infusion of ANP or nitroprusside in the maturing rat. Am J Physiol. 1992 Mar;262(3 Pt 2):F417–F424. doi: 10.1152/ajprenal.1992.262.3.F417. [DOI] [PubMed] [Google Scholar]
- Conger J. D., Robinette J. B., Hammond W. S. Differences in vascular reactivity in models of ischemic acute renal failure. Kidney Int. 1991 Jun;39(6):1087–1097. doi: 10.1038/ki.1991.138. [DOI] [PubMed] [Google Scholar]
- Endoh M., Takanashi M. Differential inhibitory action of phorbol-12,13-dibutyrate on the positive inotropic effect of endothelin-1 and Bay K 8644 in the isolated rabbit papillary muscle. J Cardiovasc Pharmacol. 1991;17 (Suppl 7):S165–S168. doi: 10.1097/00005344-199100177-00046. [DOI] [PubMed] [Google Scholar]
- Förstermann U., Schmidt H. H., Pollock J. S., Sheng H., Mitchell J. A., Warner T. D., Nakane M., Murad F. Isoforms of nitric oxide synthase. Characterization and purification from different cell types. Biochem Pharmacol. 1991 Oct 24;42(10):1849–1857. doi: 10.1016/0006-2952(91)90581-o. [DOI] [PubMed] [Google Scholar]
- Hecker M., Mitchell J. A., Harris H. J., Katsura M., Thiemermann C., Vane J. R. Endothelial cells metabolize NG-monomethyl-L-arginine to L-citrulline and subsequently to L-arginine. Biochem Biophys Res Commun. 1990 Mar 30;167(3):1037–1043. doi: 10.1016/0006-291x(90)90627-y. [DOI] [PubMed] [Google Scholar]
- Hecker M., Siegle I., Macarthur H., Sessa W. C., Vane J. R. Role of intracellular thiols in release of EDRF from cultured endothelial cells. Am J Physiol. 1992 Mar;262(3 Pt 2):H888–H896. doi: 10.1152/ajpheart.1992.262.3.H888. [DOI] [PubMed] [Google Scholar]
- Holtz J., Goetz R. M. Peptides in coronary circulation: basis for therapeutic strategies. Eur Heart J. 1991 Dec;12 (Suppl F):112–120. doi: 10.1093/eurheartj/12.suppl_f.112. [DOI] [PubMed] [Google Scholar]
- Julou-Schaeffer G., Gray G. A., Fleming I., Schott C., Parratt J. R., Stoclet J. C. Loss of vascular responsiveness induced by endotoxin involves L-arginine pathway. Am J Physiol. 1990 Oct;259(4 Pt 2):H1038–H1043. doi: 10.1152/ajpheart.1990.259.4.H1038. [DOI] [PubMed] [Google Scholar]
- Kashgarian M., Siegel N. J., Ries A. L., DiMeola H. J., Hayslett J. P. Hemodynamic aspects in development and recovery phases of experimental postischemic acute renal failure. Kidney Int Suppl. 1976 Oct;6:S160–S168. [PubMed] [Google Scholar]
- Kiyomoto H., Matsuo H., Tamaki T., Aki Y., Hong H., Iwao H., Abe Y. Effect of L-NG-nitro-arginine, inhibitor of nitric oxide synthesis, on autoregulation of renal blood flow in dogs. Jpn J Pharmacol. 1992 Feb;58(2):147–155. doi: 10.1254/jjp.58.147. [DOI] [PubMed] [Google Scholar]
- Knowles R. G., Salter M., Brooks S. L., Moncada S. Anti-inflammatory glucocorticoids inhibit the induction by endotoxin of nitric oxide synthase in the lung, liver and aorta of the rat. Biochem Biophys Res Commun. 1990 Nov 15;172(3):1042–1048. doi: 10.1016/0006-291x(90)91551-3. [DOI] [PubMed] [Google Scholar]
- Kon V., Badr K. F. Biological actions and pathophysiologic significance of endothelin in the kidney. Kidney Int. 1991 Jul;40(1):1–12. doi: 10.1038/ki.1991.172. [DOI] [PubMed] [Google Scholar]
- Lieberthal W., Wolf E. F., Rennke H. G., Valeri C. R., Levinsky N. G. Renal ischemia and reperfusion impair endothelium-dependent vascular relaxation. Am J Physiol. 1989 May;256(5 Pt 2):F894–F900. doi: 10.1152/ajprenal.1989.256.5.F894. [DOI] [PubMed] [Google Scholar]
- Macarthur H., Hecker M., Busse R., Vane J. R. Selective inhibition of agonist-induced but not shear stress-dependent release of endothelial autacoids by thapsigargin. Br J Pharmacol. 1993 Jan;108(1):100–105. doi: 10.1111/j.1476-5381.1993.tb13446.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marletta M. A., Yoon P. S., Iyengar R., Leaf C. D., Wishnok J. S. Macrophage oxidation of L-arginine to nitrite and nitrate: nitric oxide is an intermediate. Biochemistry. 1988 Nov 29;27(24):8706–8711. doi: 10.1021/bi00424a003. [DOI] [PubMed] [Google Scholar]
- Mitchell J. A., Sheng H., Förstermann U., Murad F. Characterization of nitric oxide synthases in non-adrenergic non-cholinergic nerve containing tissue from the rat anococcygeus muscle. Br J Pharmacol. 1991 Oct;104(2):289–291. doi: 10.1111/j.1476-5381.1991.tb12422.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moncada S., Palmer R. M., Higgs E. A. Nitric oxide: physiology, pathophysiology, and pharmacology. Pharmacol Rev. 1991 Jun;43(2):109–142. [PubMed] [Google Scholar]
- Moore P. K., al-Swayeh O. A., Chong N. W., Evans R. A., Gibson A. L-NG-nitro arginine (L-NOARG), a novel, L-arginine-reversible inhibitor of endothelium-dependent vasodilatation in vitro. Br J Pharmacol. 1990 Feb;99(2):408–412. doi: 10.1111/j.1476-5381.1990.tb14717.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliver J. A., Sciacca R. R., Pinto J., Cannon P. J. Participation of the prostaglandins in the control of renal blood flow during acute reduction of cardiac output in the dog. J Clin Invest. 1981 Jan;67(1):229–237. doi: 10.1172/JCI110018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollock J. S., Förstermann U., Mitchell J. A., Warner T. D., Schmidt H. H., Nakane M., Murad F. Purification and characterization of particulate endothelium-derived relaxing factor synthase from cultured and native bovine aortic endothelial cells. Proc Natl Acad Sci U S A. 1991 Dec 1;88(23):10480–10484. doi: 10.1073/pnas.88.23.10480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radermacher J., Förstermann U., Frölich J. C. Endothelium-derived relaxing factor influences renal vascular resistance. Am J Physiol. 1990 Jul;259(1 Pt 2):F9–17. doi: 10.1152/ajprenal.1990.259.1.F9. [DOI] [PubMed] [Google Scholar]
- Radermacher J., Klanke B., Kastner S., Haake G., Schurek H. J., Stolte H. F., Frölich J. C. Effect of arginine depletion on glomerular and tubular kidney function: studies in isolated perfused rat kidneys. Am J Physiol. 1991 Nov;261(5 Pt 2):F779–F786. doi: 10.1152/ajprenal.1991.261.5.F779. [DOI] [PubMed] [Google Scholar]
- Riley A. L. Effect of ischemia on renal blood flow in the rat. Nephron. 1978;21(2):107–113. doi: 10.1159/000181378. [DOI] [PubMed] [Google Scholar]
- Salter M., Knowles R. G., Moncada S. Widespread tissue distribution, species distribution and changes in activity of Ca(2+)-dependent and Ca(2+)-independent nitric oxide synthases. FEBS Lett. 1991 Oct 7;291(1):145–149. doi: 10.1016/0014-5793(91)81123-p. [DOI] [PubMed] [Google Scholar]
- Salvemini D., Korbut R., Anggård E., Vane J. Immediate release of a nitric oxide-like factor from bovine aortic endothelial cells by Escherichia coli lipopolysaccharide. Proc Natl Acad Sci U S A. 1990 Apr;87(7):2593–2597. doi: 10.1073/pnas.87.7.2593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobey C. G., Dalipram R. A., Dusting G. J., Woodman O. L. Impaired endothelium-dependent relaxation of dog coronary arteries after myocardial ischaemia and reperfusion: prevention by amlodipine, propranolol and allopurinol. Br J Pharmacol. 1992 Mar;105(3):557–562. doi: 10.1111/j.1476-5381.1992.tb09018.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprague R. S., Thiemermann C., Vane J. R. Endogenous endothelium-derived relaxing factor opposes hypoxic pulmonary vasoconstriction and supports blood flow to hypoxic alveoli in anesthetized rabbits. Proc Natl Acad Sci U S A. 1992 Sep 15;89(18):8711–8715. doi: 10.1073/pnas.89.18.8711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein J. H., Lifschitz M. D., Barnes L. D. Current concepts on the pathophysiology of acute renal failure. Am J Physiol. 1978 Mar;234(3):F171–F181. doi: 10.1152/ajprenal.1978.234.3.F171. [DOI] [PubMed] [Google Scholar]
- Stuehr D. J., Griffith O. W. Mammalian nitric oxide synthases. Adv Enzymol Relat Areas Mol Biol. 1992;65:287–346. doi: 10.1002/9780470123119.ch8. [DOI] [PubMed] [Google Scholar]
- Stuehr D. J., Kwon N. S., Nathan C. F., Griffith O. W., Feldman P. L., Wiseman J. N omega-hydroxy-L-arginine is an intermediate in the biosynthesis of nitric oxide from L-arginine. J Biol Chem. 1991 Apr 5;266(10):6259–6263. [PubMed] [Google Scholar]
- Thiemermann C. Biosynthesis and interaction of endothelium-derived vasoactive mediators. Eicosanoids. 1991;4(4):187–202. [PubMed] [Google Scholar]
- Thiemermann C., Mustafa M., Mester P. A., Mitchell J. A., Hecker M., Vane J. R. Inhibition of the release of endothelium-derived relaxing factor in vitro and in vivo by dipeptides containing NG-nitro-L-arginine. Br J Pharmacol. 1991 Sep;104(1):31–38. doi: 10.1111/j.1476-5381.1991.tb12380.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolins J. P., Palmer R. M., Moncada S., Raij L. Role of endothelium-derived relaxing factor in regulation of renal hemodynamic responses. Am J Physiol. 1990 Mar;258(3 Pt 2):H655–H662. doi: 10.1152/ajpheart.1990.258.3.H655. [DOI] [PubMed] [Google Scholar]
- Vane J. R., Botting R. M. Secretory functions of the vascular endothelium. J Physiol Pharmacol. 1992 Sep;43(3):195–207. [PubMed] [Google Scholar]
- Walder C. E., Thiemermann C., Vane J. R. NG-hydroxy-L-arginine prevents the haemodynamic effects of nitric oxide synthesis inhibition in the anaesthetized rat. Br J Pharmacol. 1992 Oct;107(2):476–480. doi: 10.1111/j.1476-5381.1992.tb12770.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walder C. E., Thiemermann C., Vane J. R. The involvement of endothelium-derived relaxing factor in the regulation of renal cortical blood flow in the rat. Br J Pharmacol. 1991 Apr;102(4):967–973. doi: 10.1111/j.1476-5381.1991.tb12285.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren J. B., Maltby N. H., MacCormack D., Barnes P. J. Pulmonary endothelium-derived relaxing factor is impaired in hypoxia. Clin Sci (Lond) 1989 Dec;77(6):671–676. doi: 10.1042/cs0770671. [DOI] [PubMed] [Google Scholar]
- Zatz R., de Nucci G. Effects of acute nitric oxide inhibition on rat glomerular microcirculation. Am J Physiol. 1991 Aug;261(2 Pt 2):F360–F363. doi: 10.1152/ajprenal.1991.261.2.F360. [DOI] [PubMed] [Google Scholar]
- Zembowicz A., Hecker M., Macarthur H., Sessa W. C., Vane J. R. Nitric oxide and another potent vasodilator are formed from NG-hydroxy-L-arginine by cultured endothelial cells. Proc Natl Acad Sci U S A. 1991 Dec 15;88(24):11172–11176. doi: 10.1073/pnas.88.24.11172. [DOI] [PMC free article] [PubMed] [Google Scholar]


