Abstract
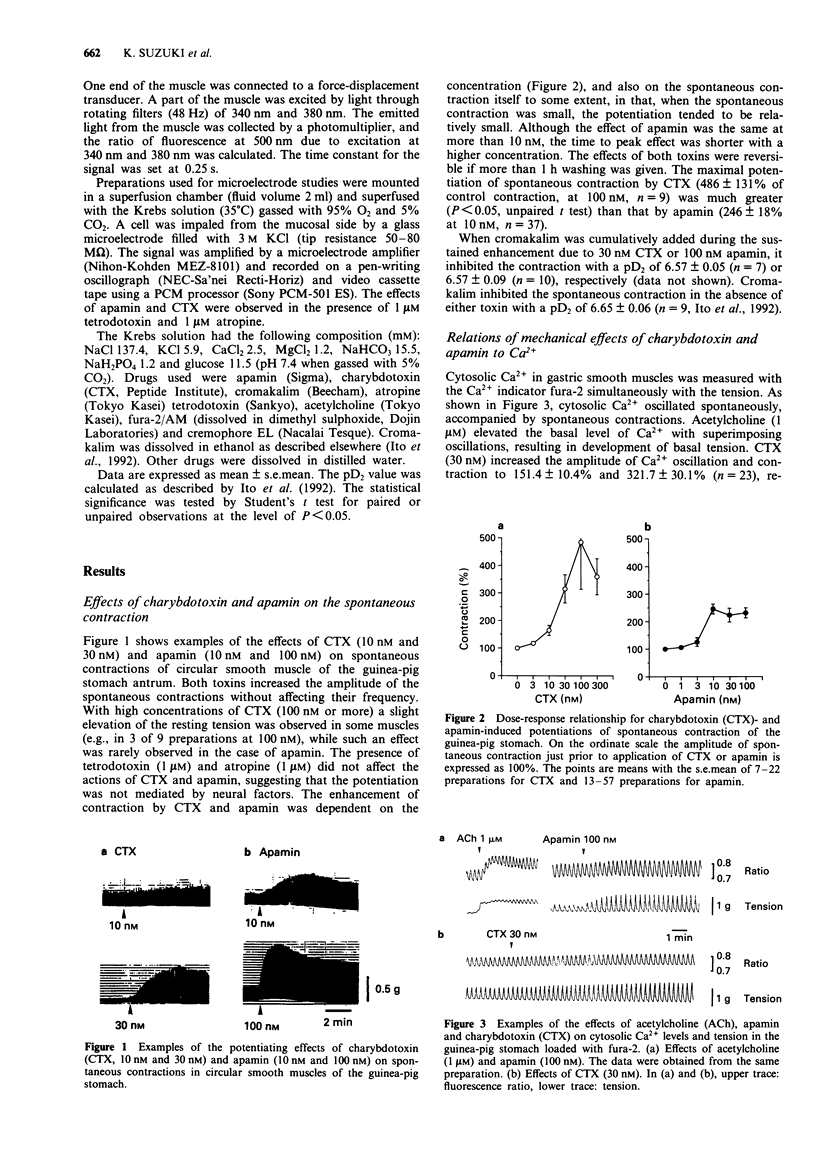
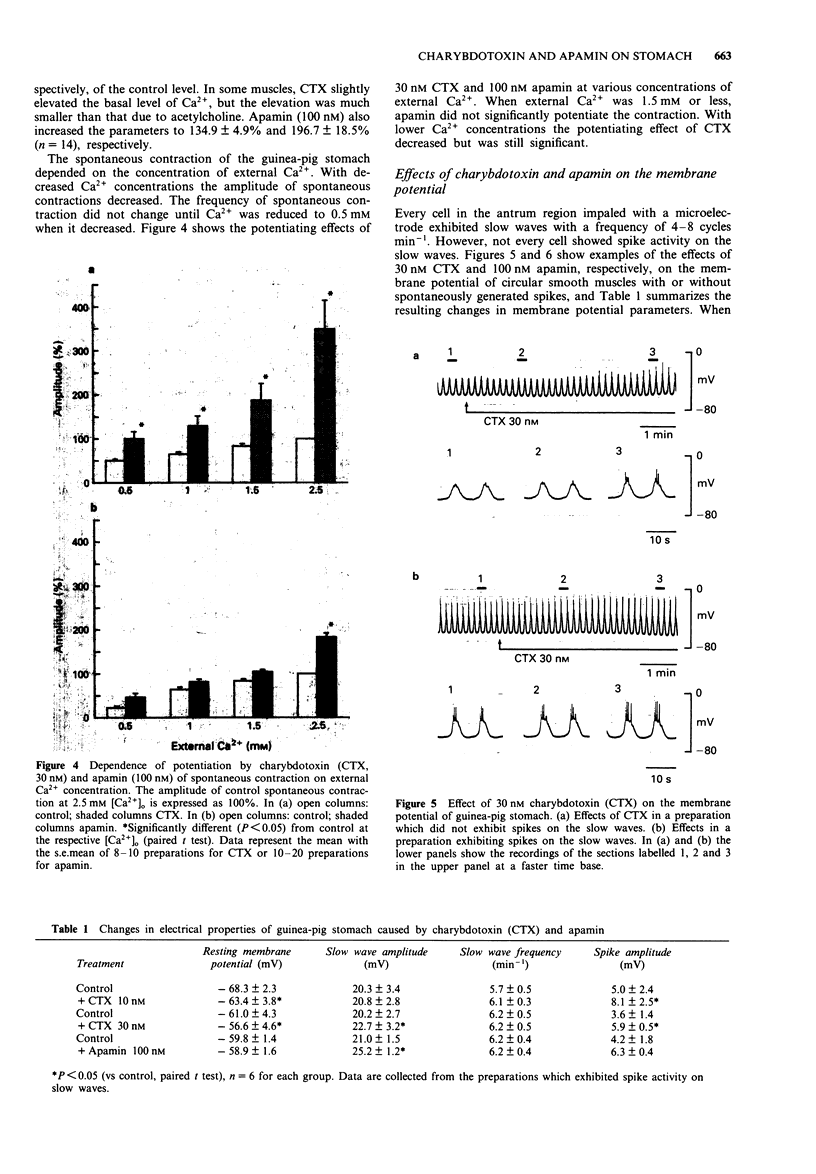
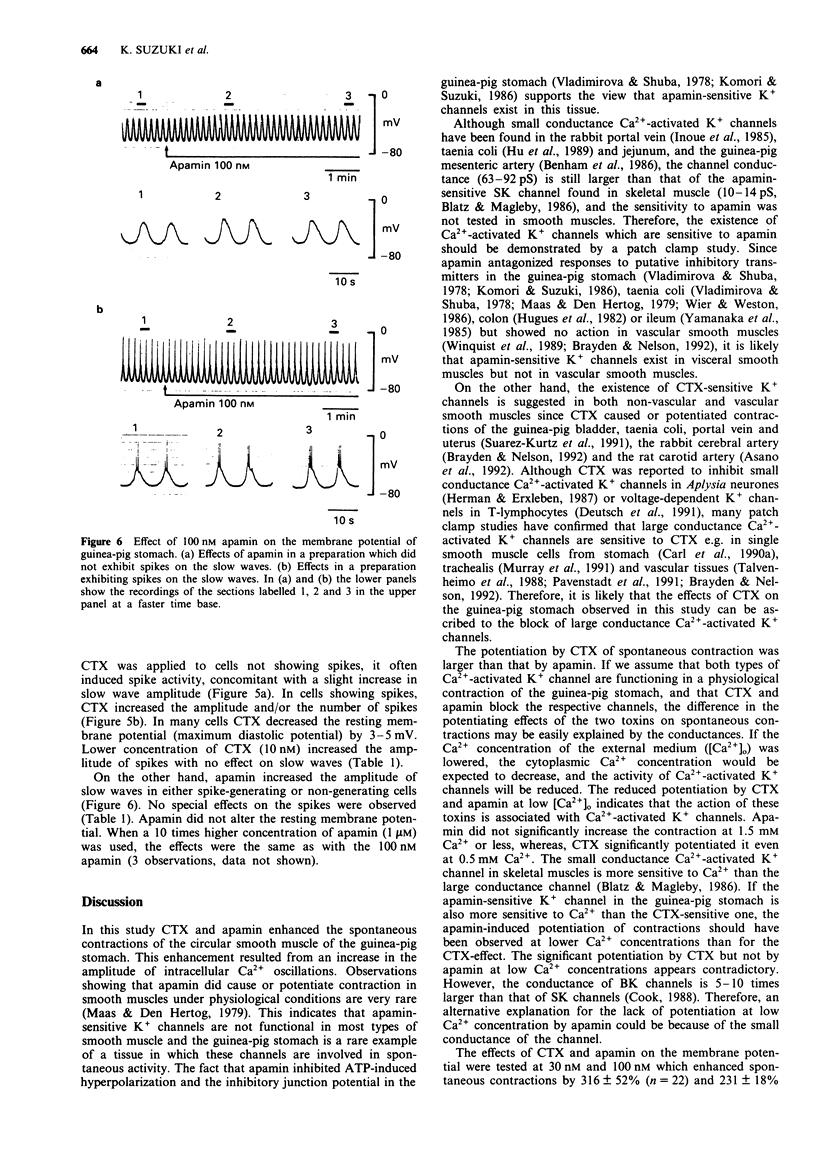
1. The effects of charybdotoxin and apamin, putative blockers of Ca(2+)-activated K+ channels, on spontaneous electrical and mechanical activity of circular smooth muscle of the guinea-pig stomach antrum were examined in the presence of 1 microM tetrodotoxin and 1 microM atropine. 2. Both charybdotoxin (> 3 nM) and apamin (> 3 nM) dose-dependently increased the amplitude of spontaneous contractions without altering their frequency. The maximum effect of charybdotoxin was much greater than that of apamin. Both toxins increased the amplitude of intracellular Ca2+ oscillations measured with fura-2. 3. When the extracellular Ca2+ concentration was lowered to 1.5 mM or less, apamin did not significantly potentiate the contractions whereas charybdotoxin still potentiated them but with less potency. 4. Charybdotoxin (30 nM) increased the amplitude of spikes and slow waves, and slightly decreased the resting membrane potential. On the other hand, apamin (100 nM) preferentially increased the slow wave amplitude with no effect on the resting membrane potential. 5. These results suggest that both toxins affect the spontaneous contraction by modifying the electrical activity and that charybdotoxin-sensitive K+ channels and apamin-sensitive ones are differently involved in the spontaneous electrical activity.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen S. L., Boyle J. P., Cortijo J., Foster R. W., Morgan G. P., Small R. C. Electrical and mechanical effects of BRL34915 in guinea-pig isolated trachealis. Br J Pharmacol. 1986 Oct;89(2):395–405. doi: 10.1111/j.1476-5381.1986.tb10273.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asano M., Masuzawa K., Matsuda T., Ito K. Increased function of Ca(2+)-activated K+ channels in the resting state of carotid arteries from spontaneously hypertensive rats. Jpn J Pharmacol. 1992;58 (Suppl 2):396P–396P. [PubMed] [Google Scholar]
- Banks B. E., Brown C., Burgess G. M., Burnstock G., Claret M., Cocks T. M., Jenkinson D. H. Apamin blocks certain neurotransmitter-induced increases in potassium permeability. Nature. 1979 Nov 22;282(5737):415–417. doi: 10.1038/282415a0. [DOI] [PubMed] [Google Scholar]
- Benham C. D., Bolton T. B., Lang R. J., Takewaki T. Calcium-activated potassium channels in single smooth muscle cells of rabbit jejunum and guinea-pig mesenteric artery. J Physiol. 1986 Feb;371:45–67. doi: 10.1113/jphysiol.1986.sp015961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blatz A. L., Magleby K. L. Single apamin-blocked Ca-activated K+ channels of small conductance in cultured rat skeletal muscle. Nature. 1986 Oct 23;323(6090):718–720. doi: 10.1038/323718a0. [DOI] [PubMed] [Google Scholar]
- Brayden J. E., Nelson M. T. Regulation of arterial tone by activation of calcium-dependent potassium channels. Science. 1992 Apr 24;256(5056):532–535. doi: 10.1126/science.1373909. [DOI] [PubMed] [Google Scholar]
- Carl A., McHale N. G., Publicover N. G., Sanders K. M. Participation of Ca2(+)-activated K+ channels in electrical activity of canine gastric smooth muscle. J Physiol. 1990 Oct;429:205–221. doi: 10.1113/jphysiol.1990.sp018252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook N. S. The pharmacology of potassium channels and their therapeutic potential. Trends Pharmacol Sci. 1988 Jan;9(1):21–28. doi: 10.1016/0165-6147(88)90238-6. [DOI] [PubMed] [Google Scholar]
- Deutsch C., Price M., Lee S., King V. F., Garcia M. L. Characterization of high affinity binding sites for charybdotoxin in human T lymphocytes. Evidence for association with the voltage-gated K+ channel. J Biol Chem. 1991 Feb 25;266(6):3668–3674. [PubMed] [Google Scholar]
- Fujii K., Foster C. D., Brading A. F., Parekh A. B. Potassium channel blockers and the effects of cromakalim on the smooth muscle of the guinea-pig bladder. Br J Pharmacol. 1990 Apr;99(4):779–785. doi: 10.1111/j.1476-5381.1990.tb13006.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermann A., Erxleben C. Charybdotoxin selectively blocks small Ca-activated K channels in Aplysia neurons. J Gen Physiol. 1987 Jul;90(1):27–47. doi: 10.1085/jgp.90.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu S. L., Yamamoto Y., Kao C. Y. The Ca2+-activated K+ channel and its functional roles in smooth muscle cells of guinea pig taenia coli. J Gen Physiol. 1989 Nov;94(5):833–847. doi: 10.1085/jgp.94.5.833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hugues M., Duval D., Schmid H., Kitabgi P., Lazdunski M., Vincent J. P. Specific binding and pharmacological interactions of apamin, the neurotoxin from bee venom, with guinea pig colon. Life Sci. 1982 Aug 2;31(5):437–443. doi: 10.1016/0024-3205(82)90328-9. [DOI] [PubMed] [Google Scholar]
- Inoue R., Kitamura K., Kuriyama H. Two Ca-dependent K-channels classified by the application of tetraethylammonium distribute to smooth muscle membranes of the rabbit portal vein. Pflugers Arch. 1985 Oct;405(3):173–179. doi: 10.1007/BF00582557. [DOI] [PubMed] [Google Scholar]
- Ito K., Kanno T., Suzuki K., Masuzawa-Ito K., Takewaki T., Ohashi H., Asano M., Suzuki H. Effects of cromakalim on the contraction and the membrane potential of the circular smooth muscle of guinea-pig stomach. Br J Pharmacol. 1992 Feb;105(2):335–340. doi: 10.1111/j.1476-5381.1992.tb14255.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komori K., Suzuki H. Distribution and properties of excitatory and inhibitory junction potentials in circular muscle of the guinea-pig stomach. J Physiol. 1986 Jan;370:339–355. doi: 10.1113/jphysiol.1986.sp015938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lydrup M. L. Role of K+ channels in spontaneous electrical and mechanical activity of smooth muscle in the guinea-pig mesotubarium. J Physiol. 1991 Feb;433:327–340. doi: 10.1113/jphysiol.1991.sp018428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maas A. J., Den Hertog A. The effect of apamin on the smooth muscle cells of the guinea-pig taenia coli. Eur J Pharmacol. 1979 Sep 15;58(2):151–156. doi: 10.1016/0014-2999(79)90006-2. [DOI] [PubMed] [Google Scholar]
- Miller C., Moczydlowski E., Latorre R., Phillips M. Charybdotoxin, a protein inhibitor of single Ca2+-activated K+ channels from mammalian skeletal muscle. Nature. 1985 Jan 24;313(6000):316–318. doi: 10.1038/313316a0. [DOI] [PubMed] [Google Scholar]
- Mitra R., Morad M. Ca2+ and Ca2+-activated K+ currents in mammalian gastric smooth muscle cells. Science. 1985 Jul 19;229(4710):269–272. doi: 10.1126/science.2409600. [DOI] [PubMed] [Google Scholar]
- Murray M. A., Berry J. L., Cook S. J., Foster R. W., Green K. A., Small R. C. Guinea-pig isolated trachealis: the effects of charybdotoxin on mechanical activity, membrane potential changes and the activity of plasmalemmal K(+)-channels. Br J Pharmacol. 1991 Jul;103(3):1814–1818. doi: 10.1111/j.1476-5381.1991.tb09868.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakao K., Inoue R., Yamanaka K., Kitamura K. Actions of quinidine and apamin on after-hyperpolarization of the spike in circular smooth muscle cells of the guinea-pig ileum. Naunyn Schmiedebergs Arch Pharmacol. 1986 Dec;334(4):508–513. doi: 10.1007/BF00569394. [DOI] [PubMed] [Google Scholar]
- Pavenstädt H., Lindeman S., Lindeman V., Späth M., Kunzelmann K., Greger R. Potassium conductance of smooth muscle cells from rabbit aorta in primary culture. Pflugers Arch. 1991 Aug;419(1):57–68. doi: 10.1007/BF00373748. [DOI] [PubMed] [Google Scholar]
- Romey G., Hugues M., Schmid-Antomarchi H., Lazdunski M. Apamin: a specific toxin to study a class of Ca2+-dependent K+ channels. J Physiol (Paris) 1984;79(4):259–264. [PubMed] [Google Scholar]
- Sato K., Ozaki H., Karaki H. Changes in cytosolic calcium level in vascular smooth muscle strip measured simultaneously with contraction using fluorescent calcium indicator fura 2. J Pharmacol Exp Ther. 1988 Jul;246(1):294–300. [PubMed] [Google Scholar]
- Suarez-Kurtz G., Garcia M. L., Kaczorowski G. J. Effects of charybdotoxin and iberiotoxin on the spontaneous motility and tonus of different guinea pig smooth muscle tissues. J Pharmacol Exp Ther. 1991 Oct;259(1):439–443. [PubMed] [Google Scholar]
- Sugg E. E., Garcia M. L., Reuben J. P., Patchett A. A., Kaczorowski G. J. Synthesis and structural characterization of charybdotoxin, a potent peptidyl inhibitor of the high conductance Ca2(+)-activated K+ channel. J Biol Chem. 1990 Nov 5;265(31):18745–18748. [PubMed] [Google Scholar]
- Toro L., Vaca L., Stefani E. Calcium-activated potassium channels from coronary smooth muscle reconstituted in lipid bilayers. Am J Physiol. 1991 Jun;260(6 Pt 2):H1779–H1789. doi: 10.1152/ajpheart.1991.260.6.H1779. [DOI] [PubMed] [Google Scholar]
- Vladimirova I. A., Shuba M. F. Vliianie strikhnina, gidrastina i apamina na sinapticheskuiu peredachu v gladkomyshechnykh kletkakh. Neirofiziologiia. 1978;10(3):295–299. [PubMed] [Google Scholar]
- Weir S. W., Weston A. H. Effect of apamin on responses to BRL 34915, nicorandil and other relaxants in the guinea-pig taenia caeci. Br J Pharmacol. 1986 May;88(1):113–120. doi: 10.1111/j.1476-5381.1986.tb09477.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winquist R. J., Heaney L. A., Wallace A. A., Baskin E. P., Stein R. B., Garcia M. L., Kaczorowski G. J. Glyburide blocks the relaxation response to BRL 34915 (cromakalim), minoxidil sulfate and diazoxide in vascular smooth muscle. J Pharmacol Exp Ther. 1989 Jan;248(1):149–156. [PubMed] [Google Scholar]
- Yamanaka K., Furukawa K., Kitamura K. The different mechanisms of action of nicorandil and adenosine triphosphate on potassium channels of circular smooth muscle of the guinea-pig small intestine. Naunyn Schmiedebergs Arch Pharmacol. 1985 Oct;331(1):96–103. doi: 10.1007/BF00498857. [DOI] [PubMed] [Google Scholar]


