Abstract
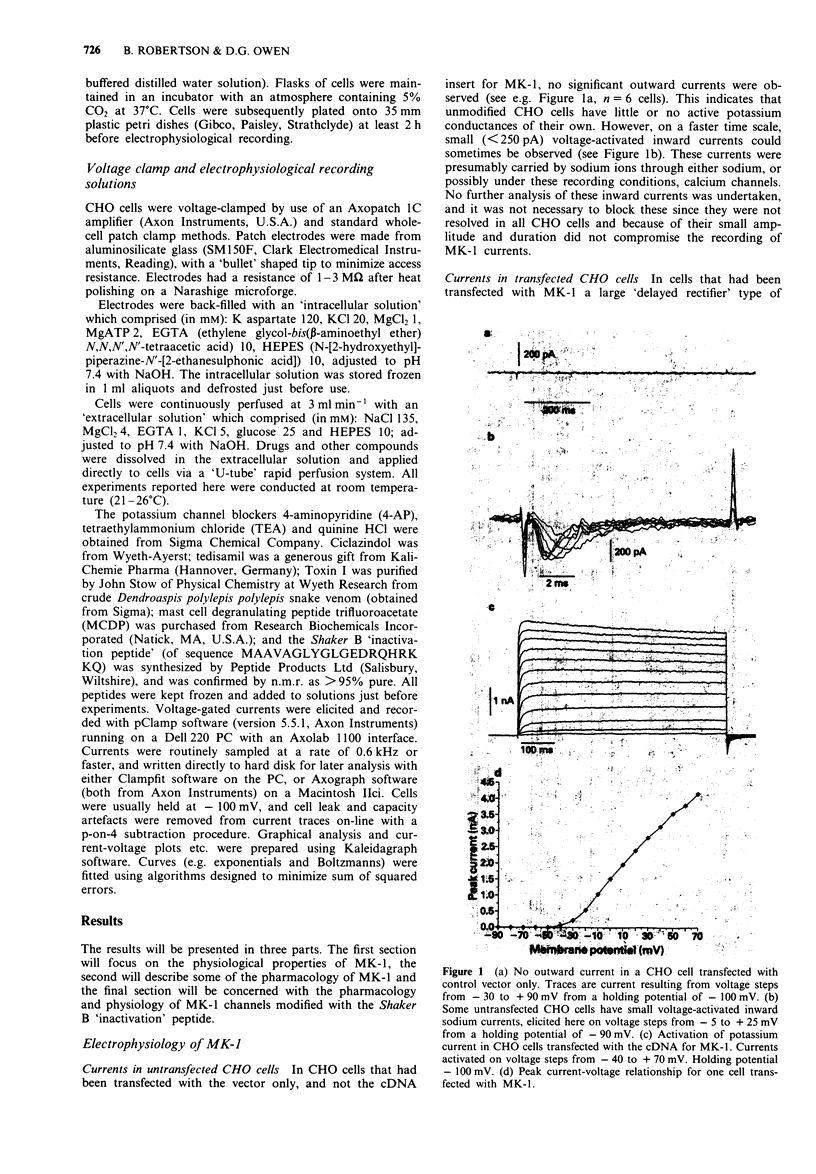
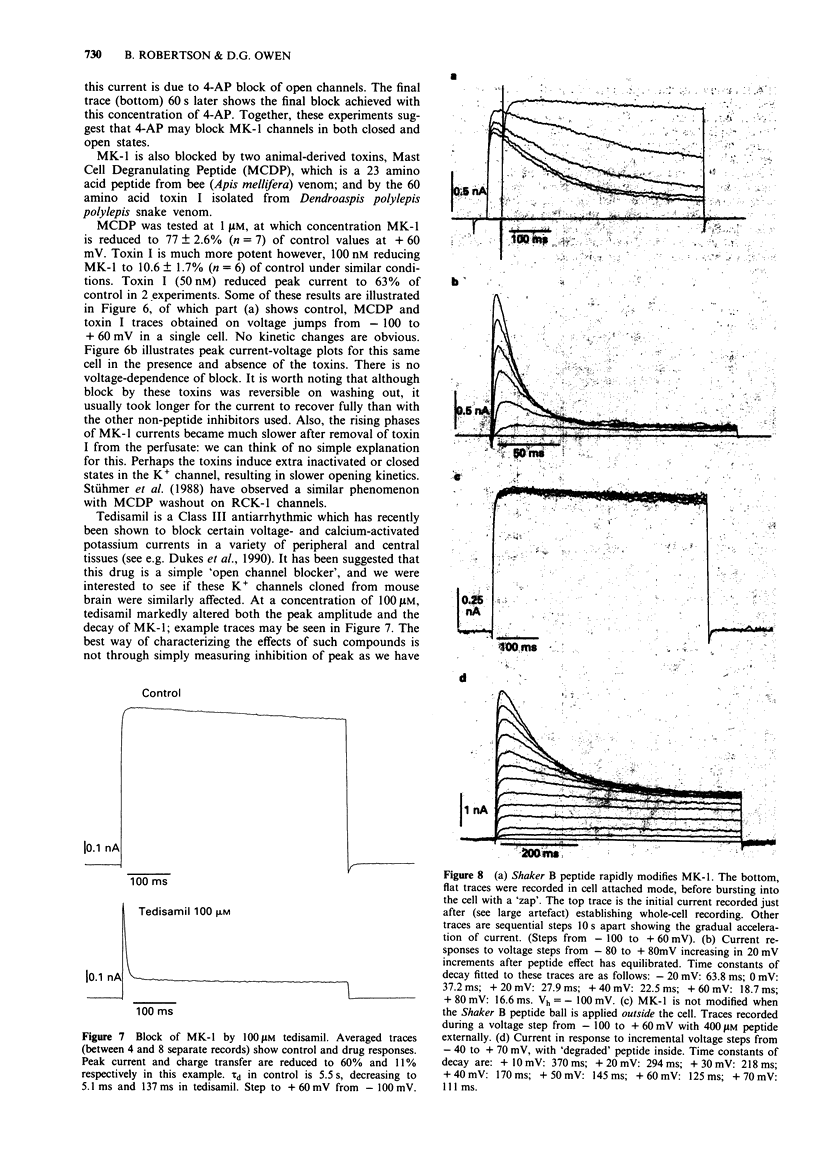
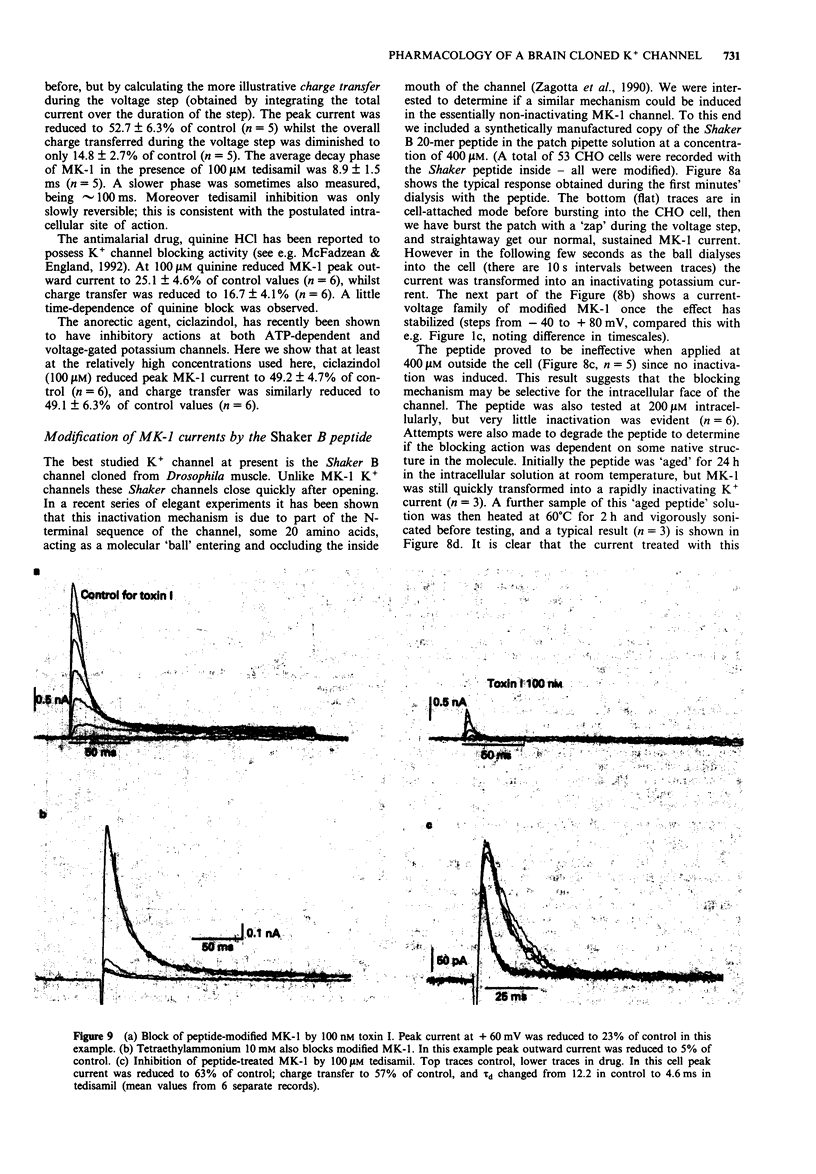
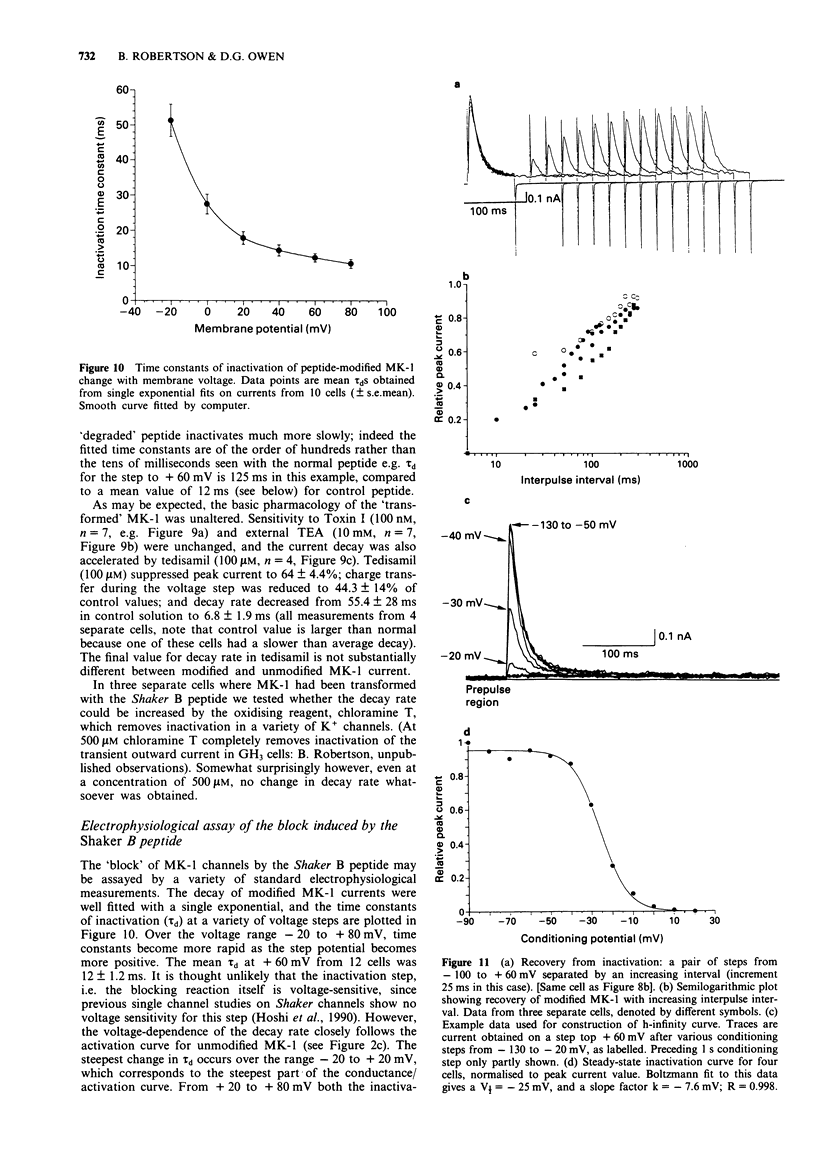
1. Chinese hamster ovary cells (CHO), maintained in cell culture, were stably transfected with DNA for the MK-1 voltage-activated potassium channel, previously cloned from a mouse brain library. 2. Voltage-activated currents were recorded by the whole cell patch clamp method. In CHO cells transfected with the vector only, there were no significant outward voltage activated currents. However, large outward voltage-activated potassium currents were always observed in those cells which had been transfected with the vector containing the DNA encoding for MK-1. 3. These potassium currents activated from -40 mV, and reversed at the potassium equilibrium potential. The half-maximal conductance of MK-1 was at -10 mV and had a slope factor of 11 mV when fitted with a Boltzmann function. There was only very slight (< 10%) inactivation of MK-1 even at very large positive voltages. 4. MK-1 was reversibly blocked by: 4-aminopyridine (4-AP, 0.1-4 mM), Toxin I 10-100 nM), mast cell degranulating peptide (1 microM), tetraethylammonium (TEA, 4-10 mM), tedisamil (100 microM), quinine (100 microM) and ciclazindol (100 microM); all applied to the outside of the cell from a 'U tube' rapid perfusion system. 4-AP may block closed as well as open MK-1 potassium channels. 5. A synthetic 20 amino acid peptide derived from the N-terminus sequence of the Shaker B potassium channel (the 'inactivation peptide') produced dramatic inactivation of MK-1 when applied to the inside, but not the outside of the cell. Reducing peptide concentration or 'degrading' the peptide produced less inactivation. 6. The block of MK-1 by the synthetic inactivation peptide was quite different in time dependence from block by internal TEA (0.4-4 mM), which probably blocks much more quickly but less potently than the peptide.
Full text
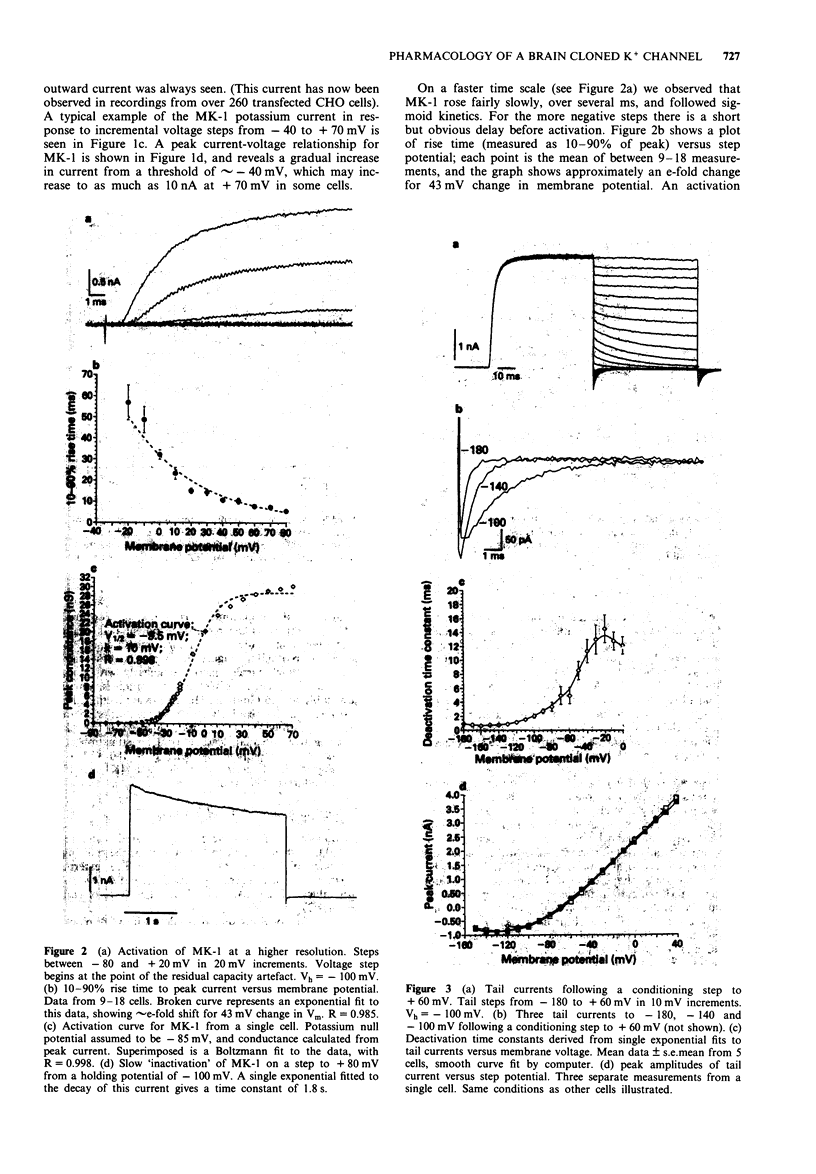
PDF
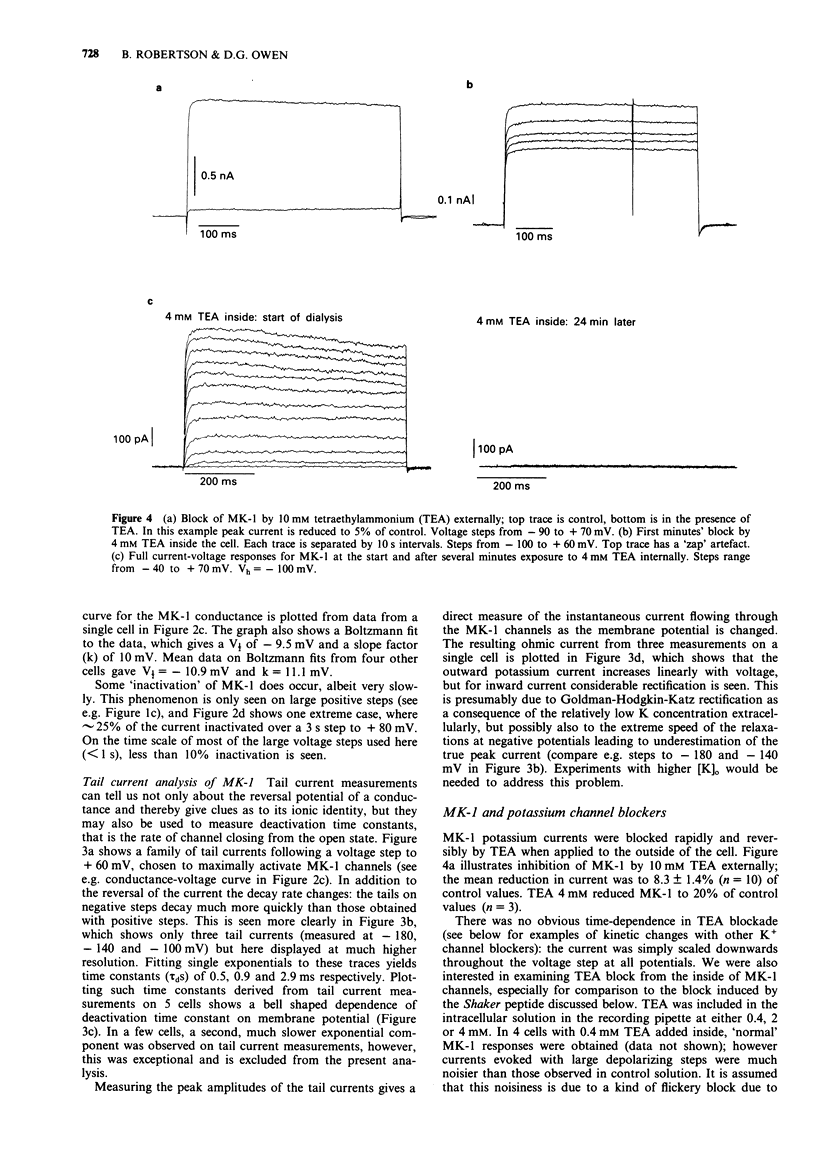
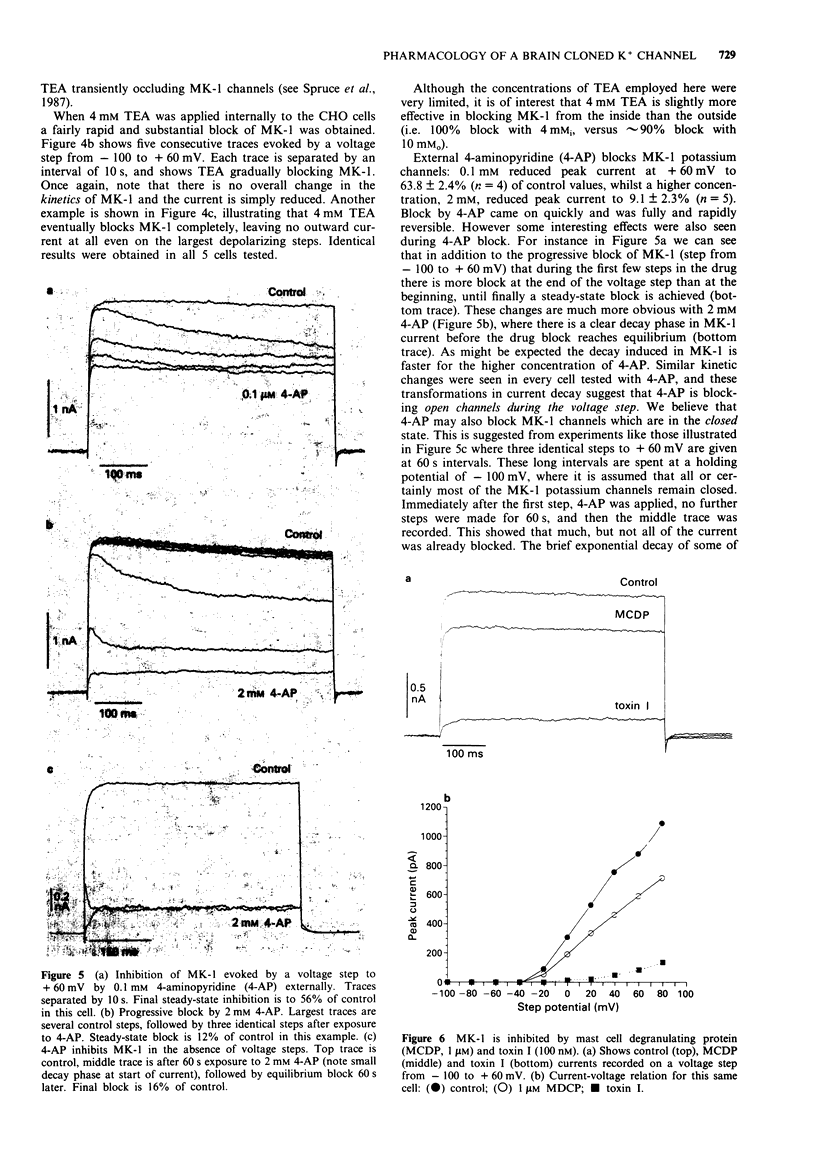



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beatch G. N., Abraham S., MacLeod B. A., Yoshida N. R., Walker M. J. Antiarrhythmic properties of tedisamil (KC8857), a putative transient outward K+ current blocker. Br J Pharmacol. 1991 Jan;102(1):13–18. doi: 10.1111/j.1476-5381.1991.tb12124.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandy K. G. Simplified gene nomenclature. Nature. 1991 Jul 4;352(6330):26–26. doi: 10.1038/352026b0. [DOI] [PubMed] [Google Scholar]
- Choi K. L., Aldrich R. W., Yellen G. Tetraethylammonium blockade distinguishes two inactivation mechanisms in voltage-activated K+ channels. Proc Natl Acad Sci U S A. 1991 Jun 15;88(12):5092–5095. doi: 10.1073/pnas.88.12.5092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christie M. J., Adelman J. P., Douglass J., North R. A. Expression of a cloned rat brain potassium channel in Xenopus oocytes. Science. 1989 Apr 14;244(4901):221–224. doi: 10.1126/science.2539643. [DOI] [PubMed] [Google Scholar]
- Demo S. D., Yellen G. The inactivation gate of the Shaker K+ channel behaves like an open-channel blocker. Neuron. 1991 Nov;7(5):743–753. doi: 10.1016/0896-6273(91)90277-7. [DOI] [PubMed] [Google Scholar]
- Dreyer F. Peptide toxins and potassium channels. Rev Physiol Biochem Pharmacol. 1990;115:93–136. [PubMed] [Google Scholar]
- Dukes I. D., Cleemann L., Morad M. Tedisamil blocks the transient and delayed rectifier K+ currents in mammalian cardiac and glial cells. J Pharmacol Exp Ther. 1990 Aug;254(2):560–569. [PubMed] [Google Scholar]
- Fatherazi S., Cook D. L. Specificity of tetraethylammonium and quinine for three K channels in insulin-secreting cells. J Membr Biol. 1991 Mar;120(2):105–114. doi: 10.1007/BF01872393. [DOI] [PubMed] [Google Scholar]
- Foster C. D., Chung S., Zagotta W. N., Aldrich R. W., Levitan I. B. A peptide derived from the Shaker B K+ channel produces short and long blocks of reconstituted Ca(2+)-dependent K+ channels. Neuron. 1992 Aug;9(2):229–236. doi: 10.1016/0896-6273(92)90162-7. [DOI] [PubMed] [Google Scholar]
- Heginbotham L., MacKinnon R. The aromatic binding site for tetraethylammonium ion on potassium channels. Neuron. 1992 Mar;8(3):483–491. doi: 10.1016/0896-6273(92)90276-j. [DOI] [PubMed] [Google Scholar]
- Hoger J. H., Walter A. E., Vance D., Yu L., Lester H. A., Davidson N. Modulation of a cloned mouse brain potassium channel. Neuron. 1991 Feb;6(2):227–236. doi: 10.1016/0896-6273(91)90358-7. [DOI] [PubMed] [Google Scholar]
- Hoshi T., Zagotta W. N., Aldrich R. W. Biophysical and molecular mechanisms of Shaker potassium channel inactivation. Science. 1990 Oct 26;250(4980):533–538. doi: 10.1126/science.2122519. [DOI] [PubMed] [Google Scholar]
- Hoshi T., Zagotta W. N., Aldrich R. W. Two types of inactivation in Shaker K+ channels: effects of alterations in the carboxy-terminal region. Neuron. 1991 Oct;7(4):547–556. doi: 10.1016/0896-6273(91)90367-9. [DOI] [PubMed] [Google Scholar]
- Isacoff E. Y., Jan Y. N., Jan L. Y. Putative receptor for the cytoplasmic inactivation gate in the Shaker K+ channel. Nature. 1991 Sep 5;353(6339):86–90. doi: 10.1038/353086a0. [DOI] [PubMed] [Google Scholar]
- Kavanaugh M. P., Varnum M. D., Osborne P. B., Christie M. J., Busch A. E., Adelman J. P., North R. A. Interaction between tetraethylammonium and amino acid residues in the pore of cloned voltage-dependent potassium channels. J Biol Chem. 1991 Apr 25;266(12):7583–7587. [PubMed] [Google Scholar]
- Klumpp D. J., Farber D. B., Bowes C., Song E. J., Pinto L. H. The potassium channel MBK1 (Kv1.1) is expressed in the mouse retina. Cell Mol Neurobiol. 1991 Dec;11(6):611–622. doi: 10.1007/BF00741449. [DOI] [PubMed] [Google Scholar]
- Koren G., Liman E. R., Logothetis D. E., Nadal-Ginard B., Hess P. Gating mechanism of a cloned potassium channel expressed in frog oocytes and mammalian cells. Neuron. 1990 Jan;4(1):39–51. doi: 10.1016/0896-6273(90)90442-i. [DOI] [PubMed] [Google Scholar]
- McFadzean I., England S. Properties of the inactivating outward current in single smooth muscle cells isolated from the rat anococcygeus. Pflugers Arch. 1992 Jun;421(2-3):117–124. doi: 10.1007/BF00374817. [DOI] [PubMed] [Google Scholar]
- McLarnon J. G., Wang X. P. Actions of cardiac drugs on a calcium-dependent potassium channel in hippocampal neurons. Mol Pharmacol. 1991 Apr;39(4):540–546. [PubMed] [Google Scholar]
- Newland C. F., Adelman J. P., Tempel B. L., Almers W. Repulsion between tetraethylammonium ions in cloned voltage-gated potassium channels. Neuron. 1992 May;8(5):975–982. doi: 10.1016/0896-6273(92)90212-v. [DOI] [PubMed] [Google Scholar]
- Noack T., Edwards G., Deitmer P., Greengrass P., Morita T., Andersson P. O., Criddle D., Wyllie M. G., Weston A. H. The involvement of potassium channels in the action of ciclazindol in rat portal vein. Br J Pharmacol. 1992 May;106(1):17–24. doi: 10.1111/j.1476-5381.1992.tb14286.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfründer D., Kreye V. A. Tedisamil blocks single large-conductance Ca(2+)-activated K+ channels in membrane patches from smooth muscle cells of the guinea-pig portal vein. Pflugers Arch. 1991 May;418(4):308–312. doi: 10.1007/BF00550866. [DOI] [PubMed] [Google Scholar]
- Ruppersberg J. P., Stocker M., Pongs O., Heinemann S. H., Frank R., Koenen M. Regulation of fast inactivation of cloned mammalian IK(A) channels by cysteine oxidation. Nature. 1991 Aug 22;352(6337):711–714. doi: 10.1038/352711a0. [DOI] [PubMed] [Google Scholar]
- Sheng M., Tsaur M. L., Jan Y. N., Jan L. Y. Subcellular segregation of two A-type K+ channel proteins in rat central neurons. Neuron. 1992 Aug;9(2):271–284. doi: 10.1016/0896-6273(92)90166-b. [DOI] [PubMed] [Google Scholar]
- Snyders J., Knoth K. M., Roberds S. L., Tamkun M. M. Time-, voltage-, and state-dependent block by quinidine of a cloned human cardiac potassium channel. Mol Pharmacol. 1992 Feb;41(2):322–330. [PubMed] [Google Scholar]
- Spruce A. E., Standen N. B., Stanfield P. R. The action of external tetraethylammonium ions on unitary delayed rectifier potassium channels of frog skeletal muscle. J Physiol. 1987 Dec;393:467–478. doi: 10.1113/jphysiol.1987.sp016833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stansfeld C. E., Marsh S. J., Parcej D. N., Dolly J. O., Brown D. A. Mast cell degranulating peptide and dendrotoxin selectively inhibit a fast-activating potassium current and bind to common neuronal proteins. Neuroscience. 1987 Dec;23(3):893–902. doi: 10.1016/0306-4522(87)90166-7. [DOI] [PubMed] [Google Scholar]
- Strong P. N. Potassium channel toxins. Pharmacol Ther. 1990;46(1):137–162. doi: 10.1016/0163-7258(90)90040-9. [DOI] [PubMed] [Google Scholar]
- Stühmer W., Stocker M., Sakmann B., Seeburg P., Baumann A., Grupe A., Pongs O. Potassium channels expressed from rat brain cDNA have delayed rectifier properties. FEBS Lett. 1988 Dec 19;242(1):199–206. doi: 10.1016/0014-5793(88)81015-9. [DOI] [PubMed] [Google Scholar]
- Stühmer W. Structure-function studies of voltage-gated ion channels. Annu Rev Biophys Biophys Chem. 1991;20:65–78. doi: 10.1146/annurev.bb.20.060191.000433. [DOI] [PubMed] [Google Scholar]
- Taglialatela M., Vandongen A. M., Drewe J. A., Joho R. H., Brown A. M., Kirsch G. E. Patterns of internal and external tetraethylammonium block in four homologous K+ channels. Mol Pharmacol. 1991 Aug;40(2):299–307. [PubMed] [Google Scholar]
- Tempel B. L., Jan Y. N., Jan L. Y. Cloning of a probable potassium channel gene from mouse brain. Nature. 1988 Apr 28;332(6167):837–839. doi: 10.1038/332837a0. [DOI] [PubMed] [Google Scholar]
- Toro L., Stefani E., Latorre R. Internal blockade of a Ca(2+)-activated K+ channel by Shaker B inactivating "ball" peptide. Neuron. 1992 Aug;9(2):237–245. doi: 10.1016/0896-6273(92)90163-8. [DOI] [PubMed] [Google Scholar]
- Wagoner P. K., Oxford G. S. Aminopyridines block an inactivating potassium current having slow recovery kinetics. Biophys J. 1990 Dec;58(6):1481–1489. doi: 10.1016/S0006-3495(90)82493-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S. Y., Castle N. A., Wang G. K. Identification of RBK1 potassium channels in C6 astrocytoma cells. Glia. 1992;5(2):146–153. doi: 10.1002/glia.440050209. [DOI] [PubMed] [Google Scholar]
- Zagotta W. N., Hoshi T., Aldrich R. W. Restoration of inactivation in mutants of Shaker potassium channels by a peptide derived from ShB. Science. 1990 Oct 26;250(4980):568–571. doi: 10.1126/science.2122520. [DOI] [PubMed] [Google Scholar]


