Abstract
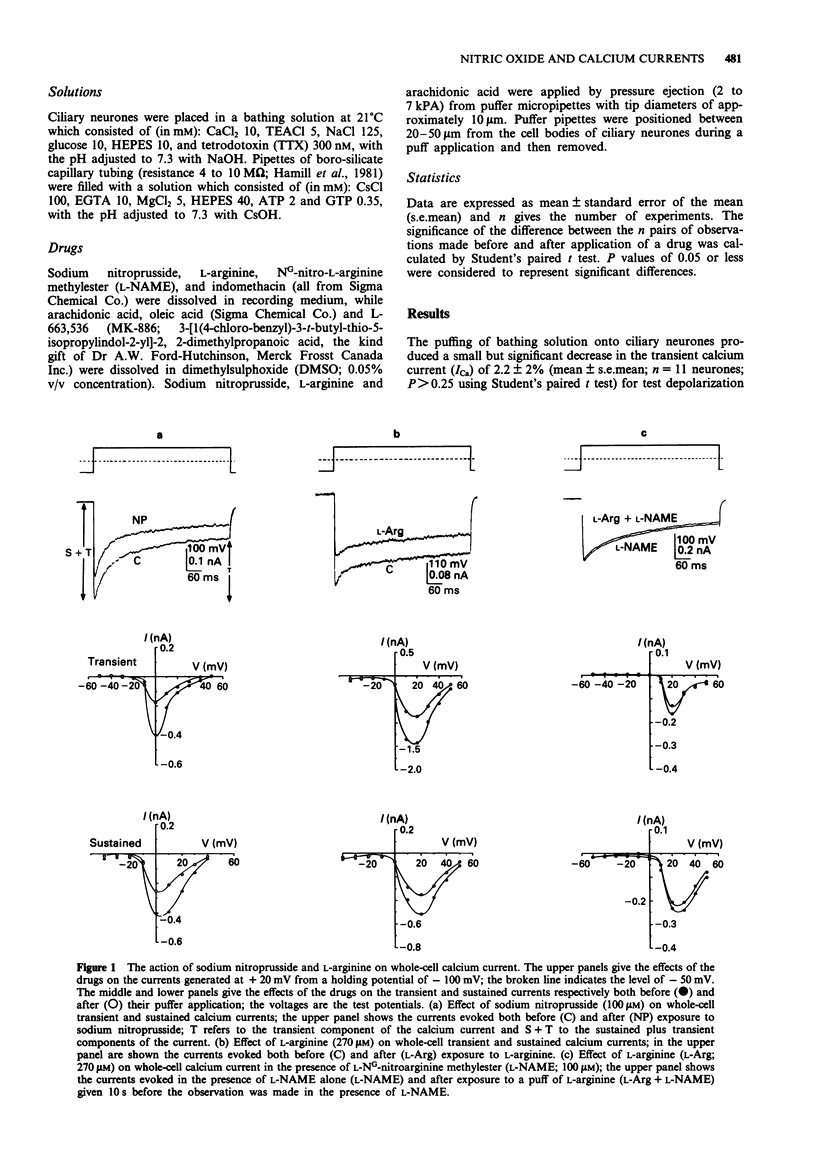
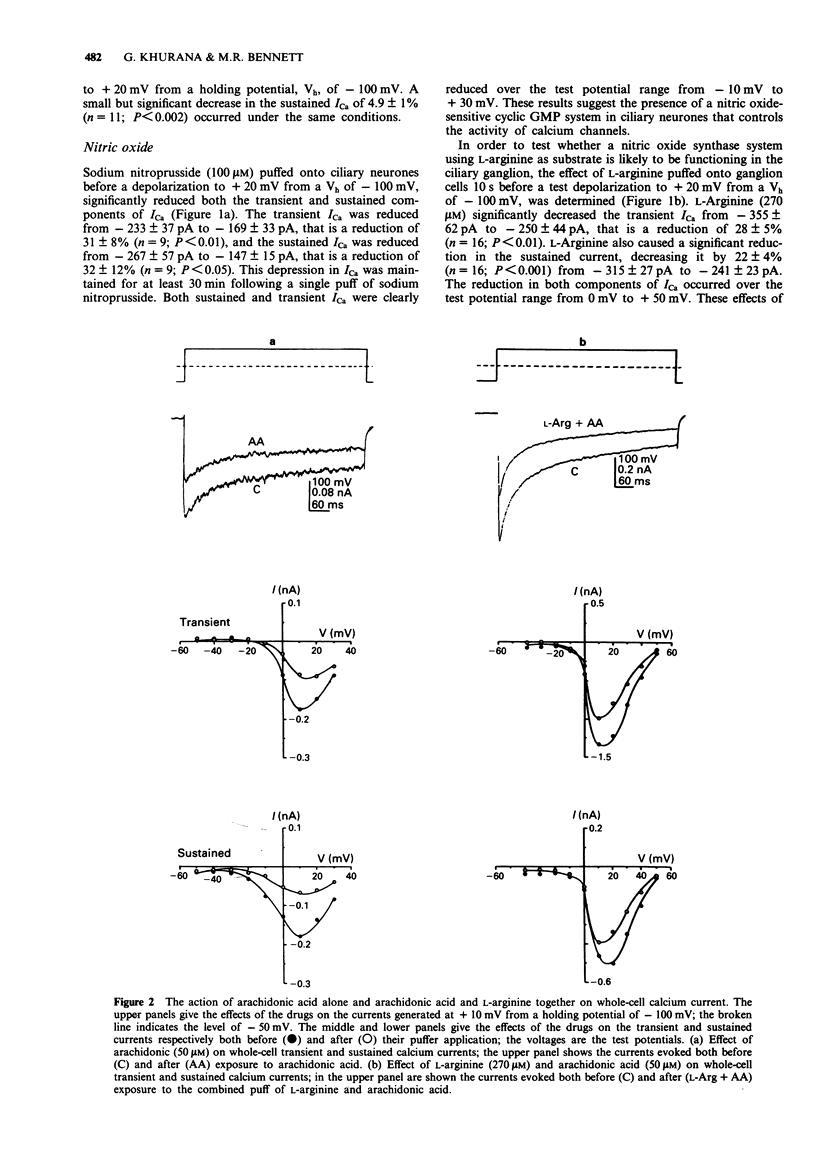
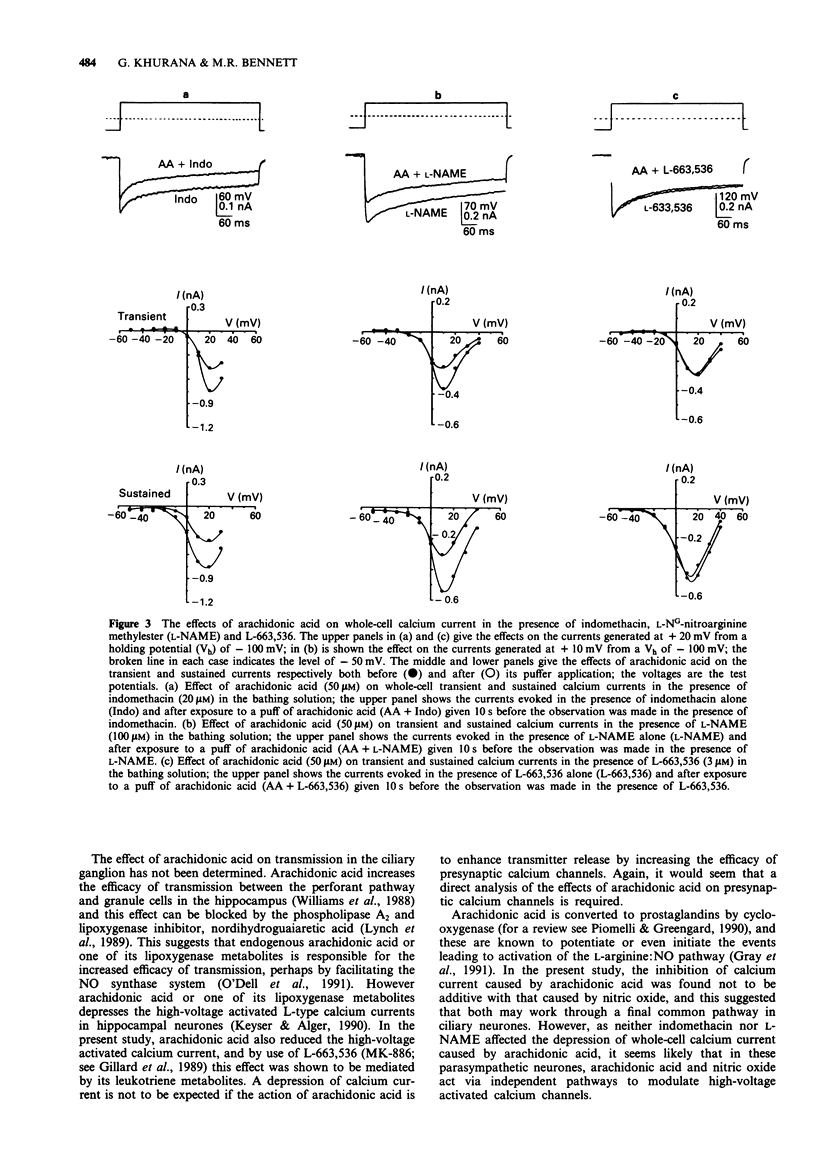
1. A study has been made of the modulation of high-voltage activated transient and sustained calcium currents in cultured neurones of avian ciliary ganglia by nitric oxide (NO) and arachidonic acid. 2. Sodium nitroprusside (100 microM) reduced the transient calcium current (ICa) on average by 31% and the sustained ICa by 32% during a test depolarization to +20 mV from a holding potential of -100 mV. This reduction was maintained for at least 30 min following a single application of sodium nitroprusside. 3. L-Arginine (270 microM) reduced the transient ICa on average by 28% and the sustained ICa by 22% and these effects were prevented by the presence of the NO-synthase competitive blocker NG-nitro-L-arginine methylester (L-NAME; 100 microM) in the bathing solution. 4. Arachidonic acid (50 microM) reduced the transient ICa on average by 28% and the sustained ICa by 33%. When added together, arachidonic acid (50 microM) and L-arginine (270 microM) produced the same effects as arachidonic acid alone. 5. Blocking the conversion of arachidonic acid to prostaglandins by addition of indomethacin (20 microM) to the bathing solution did not prevent the depression of either the transient or the sustained calcium current during application of arachidonic acid (50 microM). The effects of arachidonic acid were also not occluded by L-NAME (100 microM) when present in the bathing solution. 6. Inhibiting the biosynthesis of leukotrienes by applying L-663,536 (MK-886; 3 microM) to the bathing solution prevented the depression of both components of ICa during application of arachidonic acid (50 microM).(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bennett M. R., Burnstock G., Holman M. Transmission from intramural inhibitory nerves to the smooth muscle of the guinea-pig taenia coli. J Physiol. 1966 Feb;182(3):541–558. doi: 10.1113/jphysiol.1966.sp007836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett M. R., Kerr R., Khurana G. Adenosine modulation of calcium currents in postganglionic neurones of avian cultured ciliary ganglia. Br J Pharmacol. 1992 May;106(1):25–32. doi: 10.1111/j.1476-5381.1992.tb14287.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bredt D. S., Hwang P. M., Glatt C. E., Lowenstein C., Reed R. R., Snyder S. H. Cloned and expressed nitric oxide synthase structurally resembles cytochrome P-450 reductase. Nature. 1991 Jun 27;351(6329):714–718. doi: 10.1038/351714a0. [DOI] [PubMed] [Google Scholar]
- Bredt D. S., Hwang P. M., Snyder S. H. Localization of nitric oxide synthase indicating a neural role for nitric oxide. Nature. 1990 Oct 25;347(6295):768–770. doi: 10.1038/347768a0. [DOI] [PubMed] [Google Scholar]
- Bredt D. S., Snyder S. H. Nitric oxide, a novel neuronal messenger. Neuron. 1992 Jan;8(1):3–11. doi: 10.1016/0896-6273(92)90104-l. [DOI] [PubMed] [Google Scholar]
- Briggs C. A. Potentiation of nicotinic transmission in the rat superior cervical sympathetic ganglion: effects of cyclic GMP and nitric oxide generators. Brain Res. 1992 Feb 21;573(1):139–146. doi: 10.1016/0006-8993(92)90123-q. [DOI] [PubMed] [Google Scholar]
- Brosius D. C., Hackett J. T., Tuttle J. B. Presynaptic calcium currents evoking quantal transmission from avian ciliary ganglion neurons. Synapse. 1990;5(4):313–323. doi: 10.1002/syn.890050408. [DOI] [PubMed] [Google Scholar]
- Bult H., Boeckxstaens G. E., Pelckmans P. A., Jordaens F. H., Van Maercke Y. M., Herman A. G. Nitric oxide as an inhibitory non-adrenergic non-cholinergic neurotransmitter. Nature. 1990 May 24;345(6273):346–347. doi: 10.1038/345346a0. [DOI] [PubMed] [Google Scholar]
- Clapp L. H., Gurney A. M. Modulation of calcium movements by nitroprusside in isolated vascular smooth muscle cells. Pflugers Arch. 1991 Jun;418(5):462–470. doi: 10.1007/BF00497774. [DOI] [PubMed] [Google Scholar]
- Dawson T. M., Bredt D. S., Fotuhi M., Hwang P. M., Snyder S. H. Nitric oxide synthase and neuronal NADPH diaphorase are identical in brain and peripheral tissues. Proc Natl Acad Sci U S A. 1991 Sep 1;88(17):7797–7801. doi: 10.1073/pnas.88.17.7797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doerner D., Alger B. E. Cyclic GMP depresses hippocampal Ca2+ current through a mechanism independent of cGMP-dependent protein kinase. Neuron. 1988 Oct;1(8):693–699. doi: 10.1016/0896-6273(88)90168-7. [DOI] [PubMed] [Google Scholar]
- Fox A. P., Nowycky M. C., Tsien R. W. Kinetic and pharmacological properties distinguishing three types of calcium currents in chick sensory neurones. J Physiol. 1987 Dec;394:149–172. doi: 10.1113/jphysiol.1987.sp016864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox A. P., Nowycky M. C., Tsien R. W. Single-channel recordings of three types of calcium channels in chick sensory neurones. J Physiol. 1987 Dec;394:173–200. doi: 10.1113/jphysiol.1987.sp016865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garthwaite J. Glutamate, nitric oxide and cell-cell signalling in the nervous system. Trends Neurosci. 1991 Feb;14(2):60–67. doi: 10.1016/0166-2236(91)90022-m. [DOI] [PubMed] [Google Scholar]
- Gillard J., Ford-Hutchinson A. W., Chan C., Charleson S., Denis D., Foster A., Fortin R., Leger S., McFarlane C. S., Morton H. L-663,536 (MK-886) (3-[1-(4-chlorobenzyl)-3-t-butyl-thio-5-isopropylindol-2-yl]-2,2 - dimethylpropanoic acid), a novel, orally active leukotriene biosynthesis inhibitor. Can J Physiol Pharmacol. 1989 May;67(5):456–464. doi: 10.1139/y89-073. [DOI] [PubMed] [Google Scholar]
- Gray G. A., Schott C., Julou-Schaeffer G., Fleming I., Parratt J. R., Stoclet J. C. The effect of inhibitors of the L-arginine/nitric oxide pathway on endotoxin-induced loss of vascular responsiveness in anaesthetized rats. Br J Pharmacol. 1991 May;103(1):1218–1224. doi: 10.1111/j.1476-5381.1991.tb12327.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Hope B. T., Michael G. J., Knigge K. M., Vincent S. R. Neuronal NADPH diaphorase is a nitric oxide synthase. Proc Natl Acad Sci U S A. 1991 Apr 1;88(7):2811–2814. doi: 10.1073/pnas.88.7.2811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keyser D. O., Alger B. E. Arachidonic acid modulates hippocampal calcium current via protein kinase C and oxygen radicals. Neuron. 1990 Oct;5(4):545–553. doi: 10.1016/0896-6273(90)90092-t. [DOI] [PubMed] [Google Scholar]
- Li C. G., Rand M. J. Nitric oxide and vasoactive intestinal polypeptide mediate non-adrenergic, non-cholinergic inhibitory transmission to smooth muscle of the rat gastric fundus. Eur J Pharmacol. 1990 Dec 4;191(3):303–309. doi: 10.1016/0014-2999(90)94162-q. [DOI] [PubMed] [Google Scholar]
- Lynch M. A., Errington M. L., Bliss T. V. Nordihydroguaiaretic acid blocks the synaptic component of long-term potentiation and the associated increases in release of glutamate and arachidonate: an in vivo study in the dentate gyrus of the rat. Neuroscience. 1989;30(3):693–701. doi: 10.1016/0306-4522(89)90162-0. [DOI] [PubMed] [Google Scholar]
- Moncada S., Palmer R. M., Higgs E. A. Nitric oxide: physiology, pathophysiology, and pharmacology. Pharmacol Rev. 1991 Jun;43(2):109–142. [PubMed] [Google Scholar]
- O'Dell T. J., Hawkins R. D., Kandel E. R., Arancio O. Tests of the roles of two diffusible substances in long-term potentiation: evidence for nitric oxide as a possible early retrograde messenger. Proc Natl Acad Sci U S A. 1991 Dec 15;88(24):11285–11289. doi: 10.1073/pnas.88.24.11285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piomelli D., Greengard P. Lipoxygenase metabolites of arachidonic acid in neuronal transmembrane signalling. Trends Pharmacol Sci. 1990 Sep;11(9):367–373. doi: 10.1016/0165-6147(90)90182-8. [DOI] [PubMed] [Google Scholar]
- Piomelli D., Volterra A., Dale N., Siegelbaum S. A., Kandel E. R., Schwartz J. H., Belardetti F. Lipoxygenase metabolites of arachidonic acid as second messengers for presynaptic inhibition of Aplysia sensory cells. Nature. 1987 Jul 2;328(6125):38–43. doi: 10.1038/328038a0. [DOI] [PubMed] [Google Scholar]
- Schroeder J. E., Fischbach P. S., Mamo M., McCleskey E. W. Two components of high-threshold Ca2+ current inactivate by different mechanisms. Neuron. 1990 Oct;5(4):445–452. doi: 10.1016/0896-6273(90)90083-r. [DOI] [PubMed] [Google Scholar]
- Scott R. H., Pearson H. A., Dolphin A. C. Aspects of vertebrate neuronal voltage-activated calcium currents and their regulation. Prog Neurobiol. 1991;36(6):485–520. doi: 10.1016/0301-0082(91)90014-r. [DOI] [PubMed] [Google Scholar]
- Sher E., Clementi F. Omega-conotoxin-sensitive voltage-operated calcium channels in vertebrate cells. Neuroscience. 1991;42(2):301–307. doi: 10.1016/0306-4522(91)90376-y. [DOI] [PubMed] [Google Scholar]
- Stanley E. F., Goping G. Characterization of a calcium current in a vertebrate cholinergic presynaptic nerve terminal. J Neurosci. 1991 Apr;11(4):985–993. doi: 10.1523/JNEUROSCI.11-04-00985.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanley E. F. Single calcium channels on a cholinergic presynaptic nerve terminal. Neuron. 1991 Oct;7(4):585–591. doi: 10.1016/0896-6273(91)90371-6. [DOI] [PubMed] [Google Scholar]
- Swandulla D., Carbone E., Lux H. D. Do calcium channel classifications account for neuronal calcium channel diversity? Trends Neurosci. 1991 Feb;14(2):46–51. doi: 10.1016/0166-2236(91)90018-p. [DOI] [PubMed] [Google Scholar]
- Tare M., Parkington H. C., Coleman H. A., Neild T. O., Dusting G. J. Hyperpolarization and relaxation of arterial smooth muscle caused by nitric oxide derived from the endothelium. Nature. 1990 Jul 5;346(6279):69–71. doi: 10.1038/346069a0. [DOI] [PubMed] [Google Scholar]
- Williams J. H., Errington M. L., Lynch M. A., Bliss T. V. Arachidonic acid induces a long-term activity-dependent enhancement of synaptic transmission in the hippocampus. Nature. 1989 Oct 26;341(6244):739–742. doi: 10.1038/341739a0. [DOI] [PubMed] [Google Scholar]


