Abstract
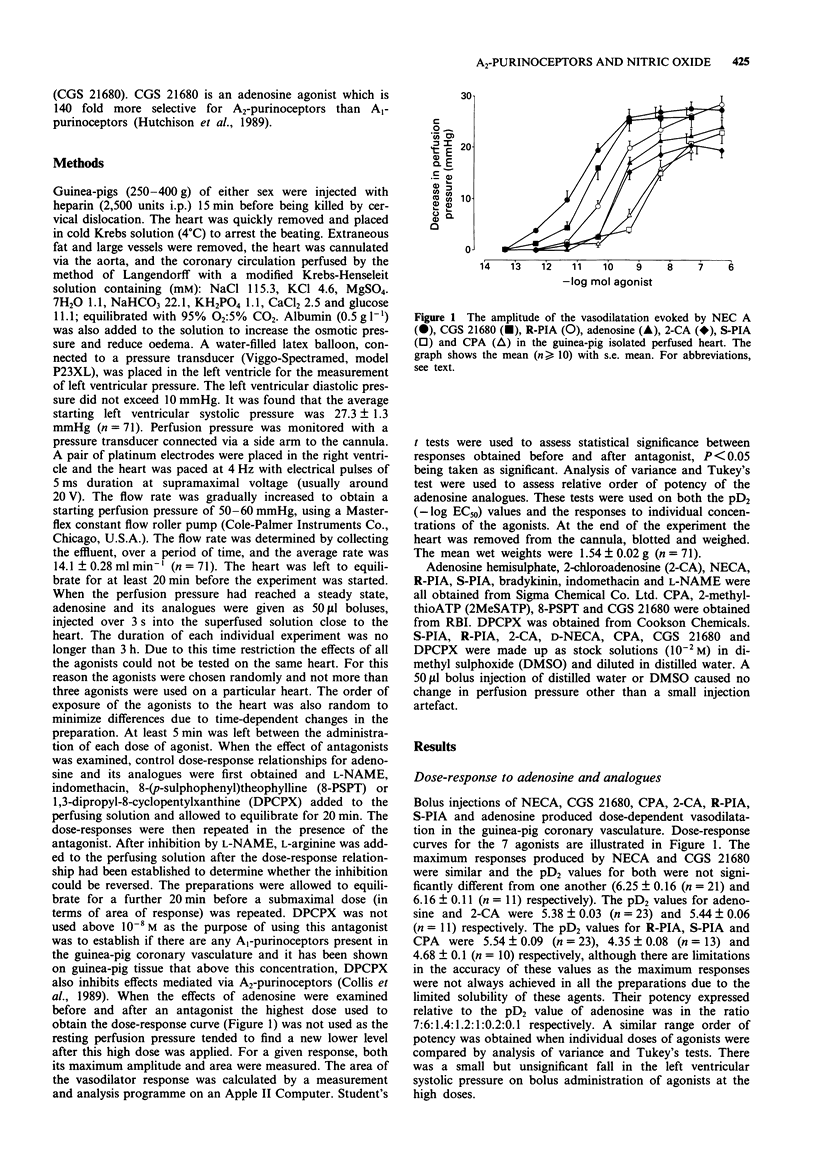
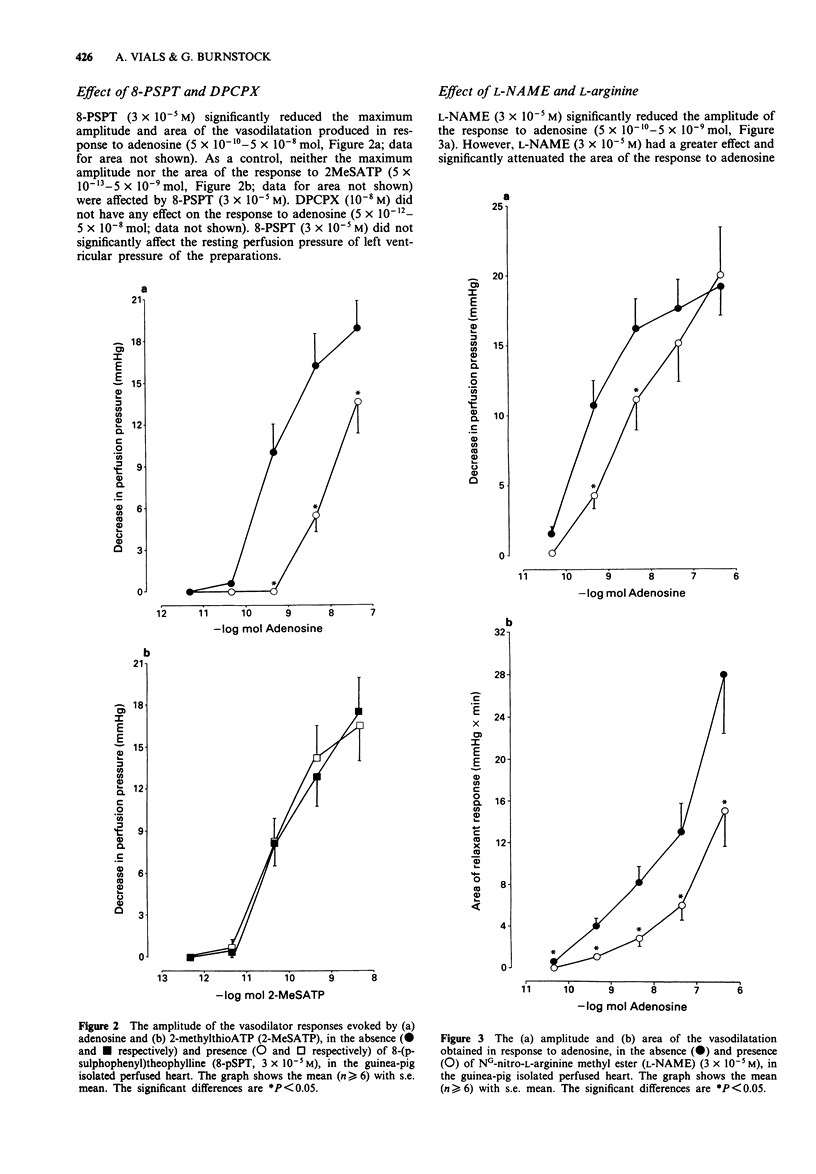
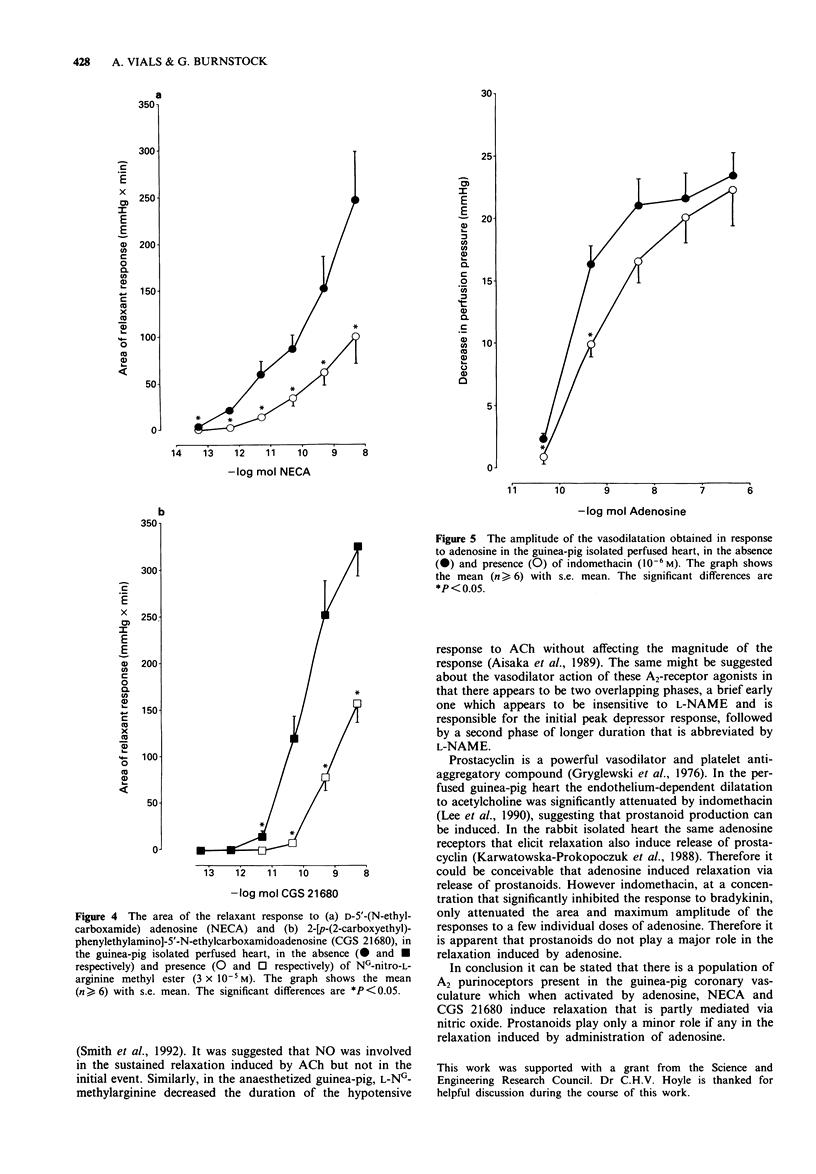
1. The Langendorff heart preparation was used to investigate the mechanism of action of the endothelium-dependent vasodilatation evoked by adenosine and its analogues in the guinea-pig coronary vasculature. 2. The relative order of potency of adenosine and its analogues in causing a reduction in perfusion pressure was D-5'-(N-ethylcarboxamide)adenosine (NECA) = 2-[p-(2-carboxyethyl)phenylethylamino]-5'-N- ethylcarboxamidoadenosine (CGS 21680)> R-N6-(2-phenylisopropyl)adenosine (R-PIA) = adenosine = 2-chloroadenosine (2-CA) > S-N6-(2-phenylisopropyl)adenosine (S-PIA) = N6-cyclopentyl-adenosine (CPA); thus suggesting the presence of A2-purinoceptors in this preparation. 3. 8-(p-Sulphophenyl)theophylline (8-PSPT; 3 x 10(-5) M) significantly reduced both the maximum amplitude and area of the vasodilatation produced in response to adenosine (5 x 10(-10) -5 x 10(-8) mol) without having any effect on the response to the P2-purinoceptor agonist, 2-methylthioATP. The relaxation induced by adenosine (5 x 10(-12) -5 x 10(-8) mol) was unaffected by the selective A1-purinoceptor antagonist 1,3-dipropyl-8-cyclopentylxanthine (DPCPX; 10(-8) M). This antagonist profile suggests that only A2-purinoceptors are present in the guinea-pig coronary vasculature. 4. The areas of the vasodilator response to adenosine (5 x 10(-10) -5 x 10(-7 mol), NECA (5 x 10(-12) -5 x 10(-7) mol) and CGS 21680 (5 x 10(-12) -5 x 10(-10) mol) were significantly reduced by NG-nitro-L-arginine methyl ester (L-NAME; 3 x 10(-5) M).(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aisaka K., Gross S. S., Griffith O. W., Levi R. L-arginine availability determines the duration of acetylcholine-induced systemic vasodilation in vivo. Biochem Biophys Res Commun. 1989 Sep 15;163(2):710–717. doi: 10.1016/0006-291x(89)92281-x. [DOI] [PubMed] [Google Scholar]
- BERNE R. M. Cardiac nucleotides in hypoxia: possible role in regulation of coronary blood flow. Am J Physiol. 1963 Feb;204:317–322. doi: 10.1152/ajplegacy.1963.204.2.317. [DOI] [PubMed] [Google Scholar]
- Balcells E., Suarez J., Rubio R. Functional role of intravascular coronary endothelial adenosine receptors. Eur J Pharmacol. 1992 Jan 7;210(1):1–9. doi: 10.1016/0014-2999(92)90644-j. [DOI] [PubMed] [Google Scholar]
- Berne R. M. The role of adenosine in the regulation of coronary blood flow. Circ Res. 1980 Dec;47(6):807–813. doi: 10.1161/01.res.47.6.807. [DOI] [PubMed] [Google Scholar]
- Bruns R. F. Adenosine receptors. Roles and pharmacology. Ann N Y Acad Sci. 1990;603:211–226. doi: 10.1111/j.1749-6632.1990.tb37674.x. [DOI] [PubMed] [Google Scholar]
- Collis M. G., Brown C. M. Adenosine relaxes the aorta by interacting with an A2 receptor and an intracellular site. Eur J Pharmacol. 1983 Dec 9;96(1-2):61–69. doi: 10.1016/0014-2999(83)90529-0. [DOI] [PubMed] [Google Scholar]
- Collis M. G., Jacobson K. A., Tomkins D. M. Apparent affinity of some 8-phenyl-substituted xanthines at adenosine receptors in guinea-pig aorta and atria. Br J Pharmacol. 1987 Sep;92(1):69–75. doi: 10.1111/j.1476-5381.1987.tb11297.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collis M. G., Stoggall S. M., Martin F. M. Apparent affinity of 1,3-dipropyl-8-cyclopentylxanthine for adenosine A1 and A2 receptors in isolated tissues from guinea-pigs. Br J Pharmacol. 1989 Aug;97(4):1274–1278. doi: 10.1111/j.1476-5381.1989.tb12589.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Des Rosiers C., Nees S. Functional evidence for the presence of adenosine A2-receptors in cultured coronary endothelial cells. Naunyn Schmiedebergs Arch Pharmacol. 1987 Jul;336(1):94–98. doi: 10.1007/BF00177757. [DOI] [PubMed] [Google Scholar]
- Deussen A., Möser G., Schrader J. Contribution of coronary endothelial cells to cardiac adenosine production. Pflugers Arch. 1986 Jun;406(6):608–614. doi: 10.1007/BF00584028. [DOI] [PubMed] [Google Scholar]
- Drury A. N., Szent-Györgyi A. The physiological activity of adenine compounds with especial reference to their action upon the mammalian heart. J Physiol. 1929 Nov 25;68(3):213–237. doi: 10.1113/jphysiol.1929.sp002608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furchgott R. F., Zawadzki J. V. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature. 1980 Nov 27;288(5789):373–376. doi: 10.1038/288373a0. [DOI] [PubMed] [Google Scholar]
- Gryglewski R. J., Bunting S., Moncada S., Flower R. J., Vane J. R. Arterial walls are protected against deposition of platelet thrombi by a substance (prostaglandin X) which they make from prostaglandin endoperoxides. Prostaglandins. 1976 Nov;12(5):685–713. doi: 10.1016/0090-6980(76)90047-2. [DOI] [PubMed] [Google Scholar]
- Haleen S. J., Steffen R. P., Hamilton H. W. PD 116,948, a highly selective A1 adenosine receptor antagonist. Life Sci. 1987 Feb 9;40(6):555–561. doi: 10.1016/0024-3205(87)90369-9. [DOI] [PubMed] [Google Scholar]
- Hutchison A. J., Webb R. L., Oei H. H., Ghai G. R., Zimmerman M. B., Williams M. CGS 21680C, an A2 selective adenosine receptor agonist with preferential hypotensive activity. J Pharmacol Exp Ther. 1989 Oct;251(1):47–55. [PubMed] [Google Scholar]
- Karwatowska-Prokopczuk E., Ciabattoni G., Wennmalm A. Effect of adenosine on the formation of prostacyclin in the rabbit isolated heart. Br J Pharmacol. 1988 Jul;94(3):721–728. doi: 10.1111/j.1476-5381.1988.tb11581.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keef K. D., Pasco J. S., Eckman D. M. Purinergic relaxation and hyperpolarization in guinea pig and rabbit coronary artery: role of the endothelium. J Pharmacol Exp Ther. 1992 Feb;260(2):592–600. [PubMed] [Google Scholar]
- Lee L., Bruner C. A., Webb R. C. Prostanoids contribute to endothelium-dependent coronary vasodilation in guinea pigs. Blood Vessels. 1990;27(6):341–351. doi: 10.1159/000158828. [DOI] [PubMed] [Google Scholar]
- Leung E., Johnston C. I., Woodcock E. A. An investigation of the receptors involved in the coronary vasodilatory effect of adenosine analogues. Clin Exp Pharmacol Physiol. 1985 Sep-Oct;12(5):515–519. doi: 10.1111/j.1440-1681.1985.tb00902.x. [DOI] [PubMed] [Google Scholar]
- Lohse M. J., Klotz K. N., Lindenborn-Fotinos J., Reddington M., Schwabe U., Olsson R. A. 8-Cyclopentyl-1,3-dipropylxanthine (DPCPX)--a selective high affinity antagonist radioligand for A1 adenosine receptors. Naunyn Schmiedebergs Arch Pharmacol. 1987 Aug;336(2):204–210. doi: 10.1007/BF00165806. [DOI] [PubMed] [Google Scholar]
- Mayer B., Schmidt K., Humbert P., Böhme E. Biosynthesis of endothelium-derived relaxing factor: a cytosolic enzyme in porcine aortic endothelial cells Ca2+-dependently converts L-arginine into an activator of soluble guanylyl cyclase. Biochem Biophys Res Commun. 1989 Oct 31;164(2):678–685. doi: 10.1016/0006-291x(89)91513-1. [DOI] [PubMed] [Google Scholar]
- Moncada S., Palmer R. M., Higgs E. A. Nitric oxide: physiology, pathophysiology, and pharmacology. Pharmacol Rev. 1991 Jun;43(2):109–142. [PubMed] [Google Scholar]
- Moncada S., Vane J. R. Pharmacology and endogenous roles of prostaglandin endoperoxides, thromboxane A2, and prostacyclin. Pharmacol Rev. 1978 Sep;30(3):293–331. [PubMed] [Google Scholar]
- Moos W. H., Szotek D. S., Bruns R. F. N6-cycloalkyladenosines. Potent, A1-selective adenosine agonists. J Med Chem. 1985 Oct;28(10):1383–1384. doi: 10.1021/jm00148a001. [DOI] [PubMed] [Google Scholar]
- Nees S., Herzog V., Becker B. F., Böck M., Des Rosiers Ch, Gerlach E. The coronary endothelium: a highly active metabolic barrier for adenosine. Basic Res Cardiol. 1985 Sep-Oct;80(5):515–529. doi: 10.1007/BF01907915. [DOI] [PubMed] [Google Scholar]
- Palmer R. M., Moncada S. A novel citrulline-forming enzyme implicated in the formation of nitric oxide by vascular endothelial cells. Biochem Biophys Res Commun. 1989 Jan 16;158(1):348–352. doi: 10.1016/s0006-291x(89)80219-0. [DOI] [PubMed] [Google Scholar]
- Palmer R. M., Rees D. D., Ashton D. S., Moncada S. L-arginine is the physiological precursor for the formation of nitric oxide in endothelium-dependent relaxation. Biochem Biophys Res Commun. 1988 Jun 30;153(3):1251–1256. doi: 10.1016/s0006-291x(88)81362-7. [DOI] [PubMed] [Google Scholar]
- Rees D. D., Palmer R. M., Schulz R., Hodson H. F., Moncada S. Characterization of three inhibitors of endothelial nitric oxide synthase in vitro and in vivo. Br J Pharmacol. 1990 Nov;101(3):746–752. doi: 10.1111/j.1476-5381.1990.tb14151.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt H. H., Nau H., Wittfoht W., Gerlach J., Prescher K. E., Klein M. M., Niroomand F., Böhme E. Arginine is a physiological precursor of endothelium-derived nitric oxide. Eur J Pharmacol. 1988 Sep 13;154(2):213–216. doi: 10.1016/0014-2999(88)90101-x. [DOI] [PubMed] [Google Scholar]
- Smith R. E., Palmer R. M., Bucknall C. A., Moncada S. Role of nitric oxide synthesis in the regulation of coronary vascular tone in the isolated perfused rabbit heart. Cardiovasc Res. 1992 May;26(5):508–512. doi: 10.1093/cvr/26.5.508. [DOI] [PubMed] [Google Scholar]
- Vane J. R. Inhibition of prostaglandin synthesis as a mechanism of action for aspirin-like drugs. Nat New Biol. 1971 Jun 23;231(25):232–235. doi: 10.1038/newbio231232a0. [DOI] [PubMed] [Google Scholar]
- Vials A., Burnstock G. Effects of nitric oxide synthase inhibitors, L-NG-nitroarginine and L-NG-nitroarginine methyl ester, on responses to vasodilators of the guinea-pig coronary vasculature. Br J Pharmacol. 1992 Oct;107(2):604–609. doi: 10.1111/j.1476-5381.1992.tb12790.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White T. D., Angus J. A. Relaxant effects of ATP and adenosine on canine large and small coronary arteries in vitro. Eur J Pharmacol. 1987 Nov 3;143(1):119–126. doi: 10.1016/0014-2999(87)90741-2. [DOI] [PubMed] [Google Scholar]
- Williams M. Purine receptors in mammalian tissues: pharmacology and functional significance. Annu Rev Pharmacol Toxicol. 1987;27:315–345. doi: 10.1146/annurev.pa.27.040187.001531. [DOI] [PubMed] [Google Scholar]


