Abstract
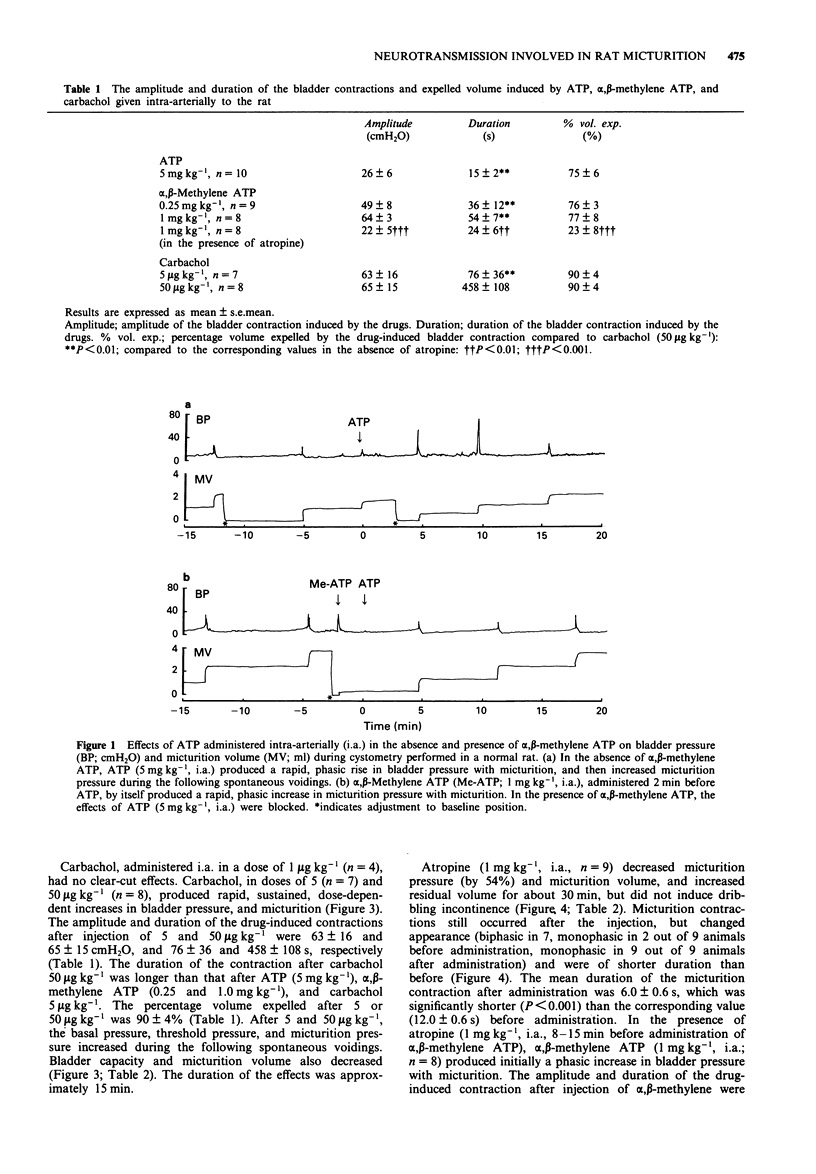
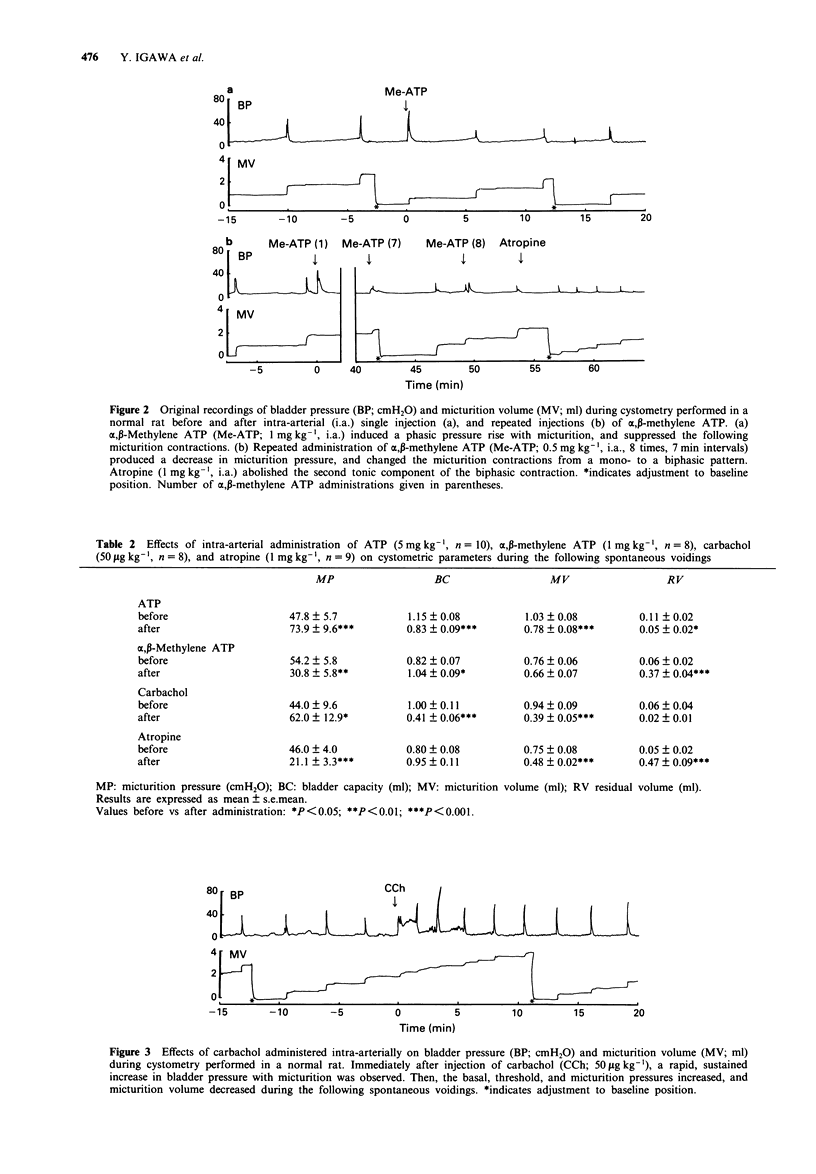
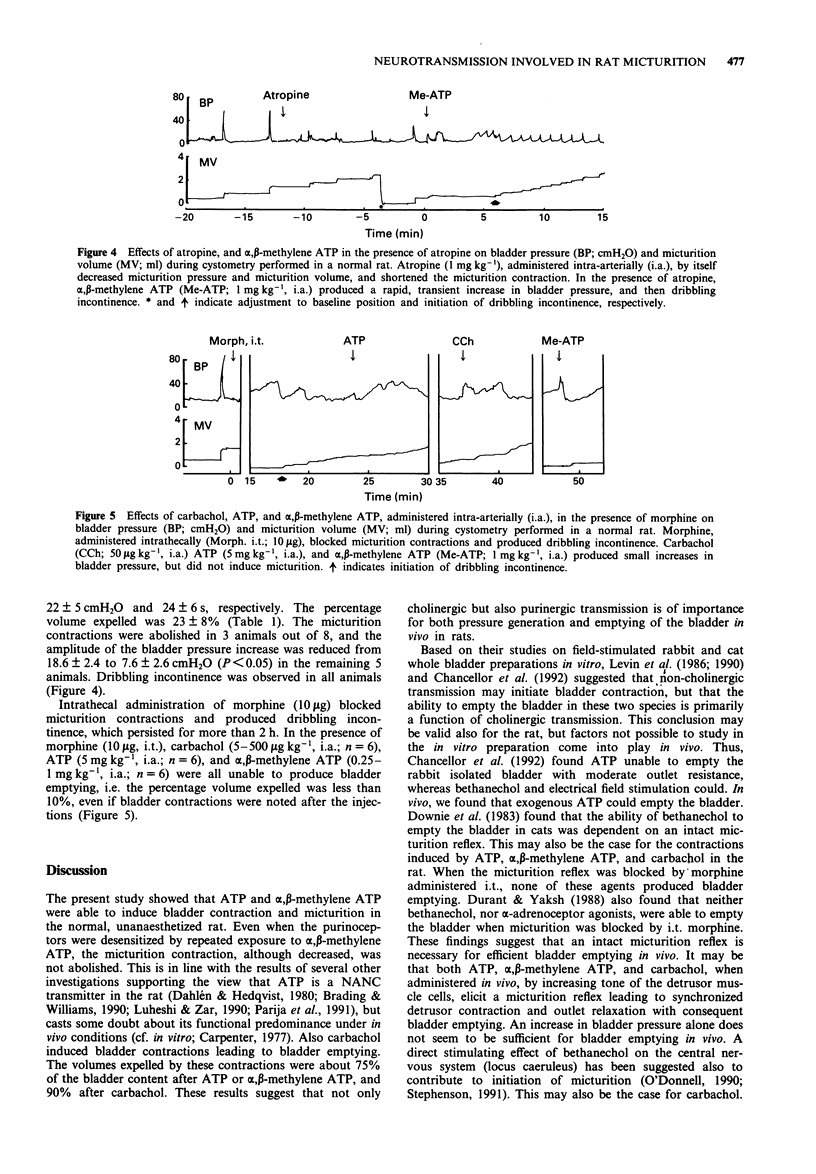
1. The cholinergic and purinergic neurotransmission involved in micturition in the normal, unanaesthetized rat was investigated by means of continuous cystometry. 2. ATP (1 and 5 mg kg-1), administered intra-arterially (i.a.) close to the bladder, produced rapid, phasic, dose-dependent increases in bladder pressure with micturition immediately after injection. The micturition pressure of the following spontaneous voidings increased, and bladder capacity, micturition volume, and residual volume decreased. Pretreatment with alpha,beta-methylene ATP (1 mg kg-1, i.a.) blocked the effects of ATP (5 mg kg-1). 3. alpha,beta-Methylene ATP (0.25, 0.5 and 1 mg kg-1, i.a.) produced rapid, phasic, increases in bladder pressure with micturition immediately after injection. The effects of alpha,beta-methylene ATP (0.25 mg kg-1, i.a.) were not affected by pretreatment with indomethacin (0.5-2 mg kg-1, i.a.). The micturition pressure of the subsequent spontaneous voidings decreased, and bladder capacity and residual volume increased. 4. Carbachol (5-50 micrograms kg-1, i.a.) produced rapid, sustained, dose-dependent increases in bladder pressure with micturition, and then increased basal pressure, threshold pressure, and micturition pressure, and decreased bladder capacity and micturition volume during the following spontaneous voidings. 5. Atropine (1 mg kg-1, i.a.) decreased micturition pressure and micturition volume, but did not induce dribbling incontinence. Micturition contractions still occurred after the injection, but changed in appearance and were of shorter duration than before. In the presence of atropine (1 mg kg-1, i.a.), alpha,beta-methylene ATP (1 mg kg-1, i.a.) produced initially a phasic increase in bladder pressure with micturition and then dribbling incontinence in all animals tested.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ambache N., Zar M. A. Non-cholinergic transmission by post-ganglionic motor neurones in the mammalian bladder. J Physiol. 1970 Oct;210(3):761–783. doi: 10.1113/jphysiol.1970.sp009240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhat M. B., Mishra S. K., Raviprakash V. Differential susceptibility of cholinergic and noncholinergic neurogenic responses to calcium channel blockers and low Ca2+ medium in rat urinary bladder. Br J Pharmacol. 1989 Apr;96(4):837–842. doi: 10.1111/j.1476-5381.1989.tb11892.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brading A. F., Mostwin J. L. Electrical and mechanical responses of guinea-pig bladder muscle to nerve stimulation. Br J Pharmacol. 1989 Dec;98(4):1083–1090. doi: 10.1111/j.1476-5381.1989.tb12651.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brading A. F., Williams J. H. Contractile responses of smooth muscle strips from rat and guinea-pig urinary bladder to transmural stimulation: effects of atropine and alpha,beta-methylene ATP. Br J Pharmacol. 1990 Mar;99(3):493–498. doi: 10.1111/j.1476-5381.1990.tb12956.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnstock G., Cocks T., Crowe R., Kasakov L. Purinergic innervation of the guinea-pig urinary bladder. Br J Pharmacol. 1978 May;63(1):125–138. doi: 10.1111/j.1476-5381.1978.tb07782.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnstock G., Cocks T., Kasakov L., Wong H. K. Direct evidence for ATP release from non-adrenergic, non-cholinergic ("purinergic") nerves in the guinea-pig taenia coli and bladder. Eur J Pharmacol. 1978 May 15;49(2):145–149. doi: 10.1016/0014-2999(78)90070-5. [DOI] [PubMed] [Google Scholar]
- Burnstock G., Dumsday B., Smythe A. Atropine resistant excitation of the urinary bladder: the possibility of transmission via nerves releasing a purine nucleotide. Br J Pharmacol. 1972 Mar;44(3):451–461. doi: 10.1111/j.1476-5381.1972.tb07283.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter F. G. Atropine resistance and muscarinic receptors in the rat urinary bladder. Br J Pharmacol. 1977 Jan;59(1):43–49. doi: 10.1111/j.1476-5381.1977.tb06975.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chancellor M. B., Kaplan S. A., Blaivas J. G. The cholinergic and purinergic components of detrusor contractility in a whole rabbit bladder model. J Urol. 1992 Sep;148(3):906–909. doi: 10.1016/s0022-5347(17)36775-7. [DOI] [PubMed] [Google Scholar]
- Choo L. K., Mitchelson F. The effect of indomethacin and adenosine 5'-triphosphate on the excitatory innervation of the rate urinary bladder. Can J Physiol Pharmacol. 1980 Sep;58(9):1042–1048. doi: 10.1139/y80-157. [DOI] [PubMed] [Google Scholar]
- Creed K. E., Ito Y., Katsuyama H. Neurotransmission in the urinary bladder of rabbits and guinea pigs. Am J Physiol. 1991 Aug;261(2 Pt 1):C271–C277. doi: 10.1152/ajpcell.1991.261.2.C271. [DOI] [PubMed] [Google Scholar]
- Dahlén S. E., Hedqvist P. ATP, beta-gamma-methylene-ATP, and adenosine inhibit non-cholinergic non-adrenergic transmission in rat urinary bladder. Acta Physiol Scand. 1980 Jun;109(2):137–142. doi: 10.1111/j.1748-1716.1980.tb06578.x. [DOI] [PubMed] [Google Scholar]
- Dean D. M., Downie J. W. Contribution of adrenergic and "purinergic" neurotransmission to contraction in rabbit detrusor. J Pharmacol Exp Ther. 1978 Nov;207(2):431–445. [PubMed] [Google Scholar]
- Durant P. A., Yaksh T. L. Drug effects on urinary bladder tone during spinal morphine-induced inhibition of the micturition reflex in unanesthetized rats. Anesthesiology. 1988 Mar;68(3):325–334. doi: 10.1097/00000542-198803000-00002. [DOI] [PubMed] [Google Scholar]
- Fujii K. Evidence for adenosine triphosphate as an excitatory transmitter in guinea-pig, rabbit and pig urinary bladder. J Physiol. 1988 Oct;404:39–52. doi: 10.1113/jphysiol.1988.sp017277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoyle C. H., Burnstock G. Atropine-resistant excitatory junction potentials in rabbit bladder are blocked by alpha,beta-methylene ATP. Eur J Pharmacol. 1985 Aug 15;114(2):239–240. doi: 10.1016/0014-2999(85)90635-1. [DOI] [PubMed] [Google Scholar]
- Hoyle C. H., Chapple C., Burnstock G. Isolated human bladder: evidence for an adenine dinucleotide acting on P2X-purinoceptors and for purinergic transmission. Eur J Pharmacol. 1989 Dec 12;174(1):115–118. doi: 10.1016/0014-2999(89)90881-9. [DOI] [PubMed] [Google Scholar]
- Kasakov L. N., Vlaskovska M. V. Profile of prostaglandins generated in the detrusor muscle of rat urinary bladder: effects of adenosine triphosphate and adenosine. Eur J Pharmacol. 1985 Jul 31;113(3):431–436. doi: 10.1016/0014-2999(85)90092-5. [DOI] [PubMed] [Google Scholar]
- Kasakov L., Burnstock G. The use of the slowly degradable analog, alpha, beta-methylene ATP, to produce desensitisation of the P2-purinoceptor: effect on non-adrenergic, non-cholinergic responses of the guinea-pig urinary bladder. Eur J Pharmacol. 1982 Dec 24;86(2):291–294. doi: 10.1016/0014-2999(82)90330-2. [DOI] [PubMed] [Google Scholar]
- Levin R. M., Longhurst P. A., Kato K., McGuire E. J., Elbadawi A., Wein A. J. Comparative physiology and pharmacology of the cat and rabbit urinary bladder. J Urol. 1990 Apr;143(4):848–852. doi: 10.1016/s0022-5347(17)40115-7. [DOI] [PubMed] [Google Scholar]
- Levin R. M., Ruggieri M. R., Wein A. J. Functional effects of the purinergic innervation of the rabbit urinary bladder. J Pharmacol Exp Ther. 1986 Feb;236(2):452–457. [PubMed] [Google Scholar]
- Luheshi G., Zar A. Purinoceptor desensitization impairs but does not abolish the non-cholinergic motor transmission in rat isolated urinary bladder. Eur J Pharmacol. 1990 Aug 28;185(2-3):203–208. doi: 10.1016/0014-2999(90)90641-i. [DOI] [PubMed] [Google Scholar]
- Maggi C. A. Omega conotoxin and prejunctional modulation of the biphasic response of the rat isolated urinary bladder to single pulse electrical field stimulation. J Auton Pharmacol. 1991 Oct;11(5):295–304. doi: 10.1111/j.1474-8673.1991.tb00253.x. [DOI] [PubMed] [Google Scholar]
- Maggi C. A., Santicioli P., Meli A. Pharmacological evidence for the existence of two components in the twitch response to field stimulation of detrusor strips from the rat urinary bladder. J Auton Pharmacol. 1985 Sep;5(3):221–229. doi: 10.1111/j.1474-8673.1985.tb00123.x. [DOI] [PubMed] [Google Scholar]
- Malmgren A., Sjögren C., Uvelius B., Mattiasson A., Andersson K. E., Andersson P. O. Cystometrical evaluation of bladder instability in rats with infravesical outflow obstruction. J Urol. 1987 Jun;137(6):1291–1294. doi: 10.1016/s0022-5347(17)44485-5. [DOI] [PubMed] [Google Scholar]
- O'Donnell P. D. Central actions of bethanechol on the urinary bladder in dogs. J Urol. 1990 Mar;143(3):634–637. doi: 10.1016/s0022-5347(17)40045-0. [DOI] [PubMed] [Google Scholar]
- Parija S. C., Raviprakash V., Mishra S. K. Adenosine- and alpha,beta-methylene ATP-induced differential inhibition of cholinergic and non-cholinergic neurogenic responses in rat urinary bladder. Br J Pharmacol. 1991 Feb;102(2):396–400. doi: 10.1111/j.1476-5381.1991.tb12185.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sibley G. N. A comparison of spontaneous and nerve-mediated activity in bladder muscle from man, pig and rabbit. J Physiol. 1984 Sep;354:431–443. doi: 10.1113/jphysiol.1984.sp015386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephenson J. D. Pharmacology of the central control of micturition. Funct Neurol. 1991 Jul-Sep;6(3):211–217. [PubMed] [Google Scholar]
- Theobald R. J., Jr The effect of arylazido aminopropionyl ATP on atropine resistant contractions of the cat urinary bladder. Life Sci. 1983 May 23;32(21):2479–2484. doi: 10.1016/0024-3205(83)90374-0. [DOI] [PubMed] [Google Scholar]


