Abstract
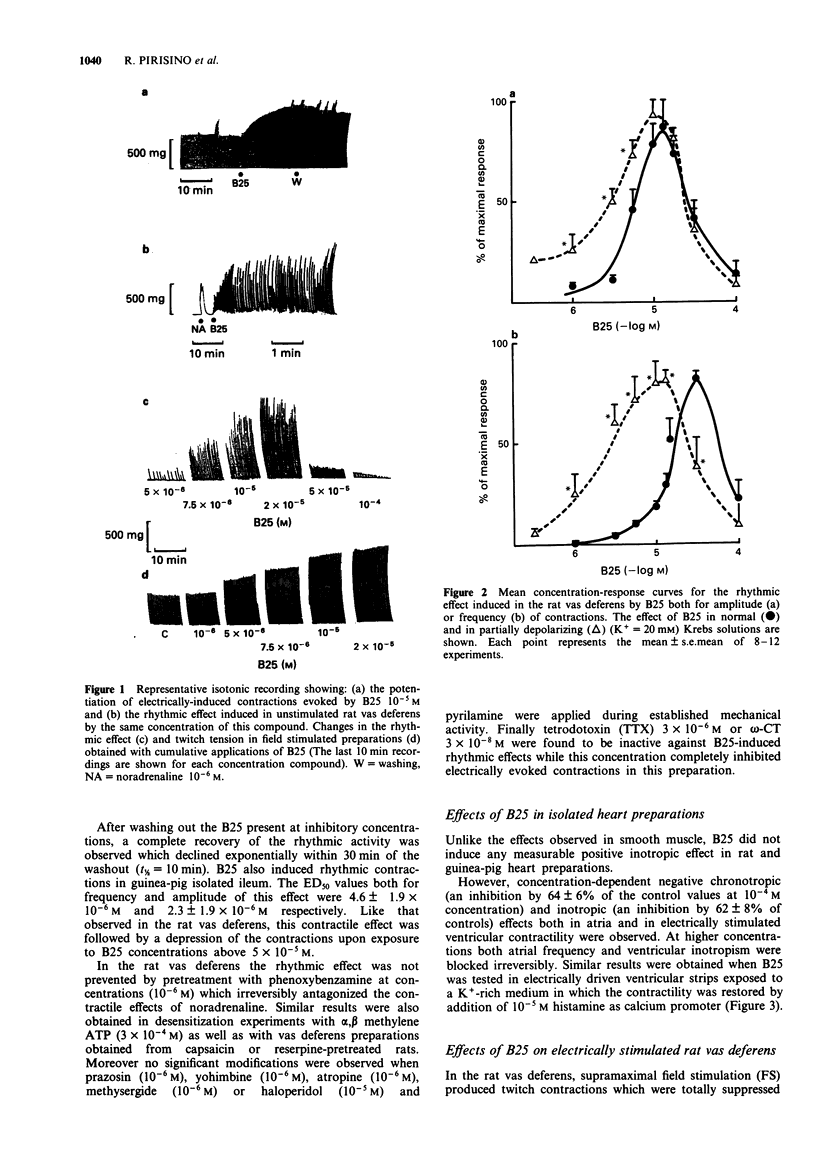
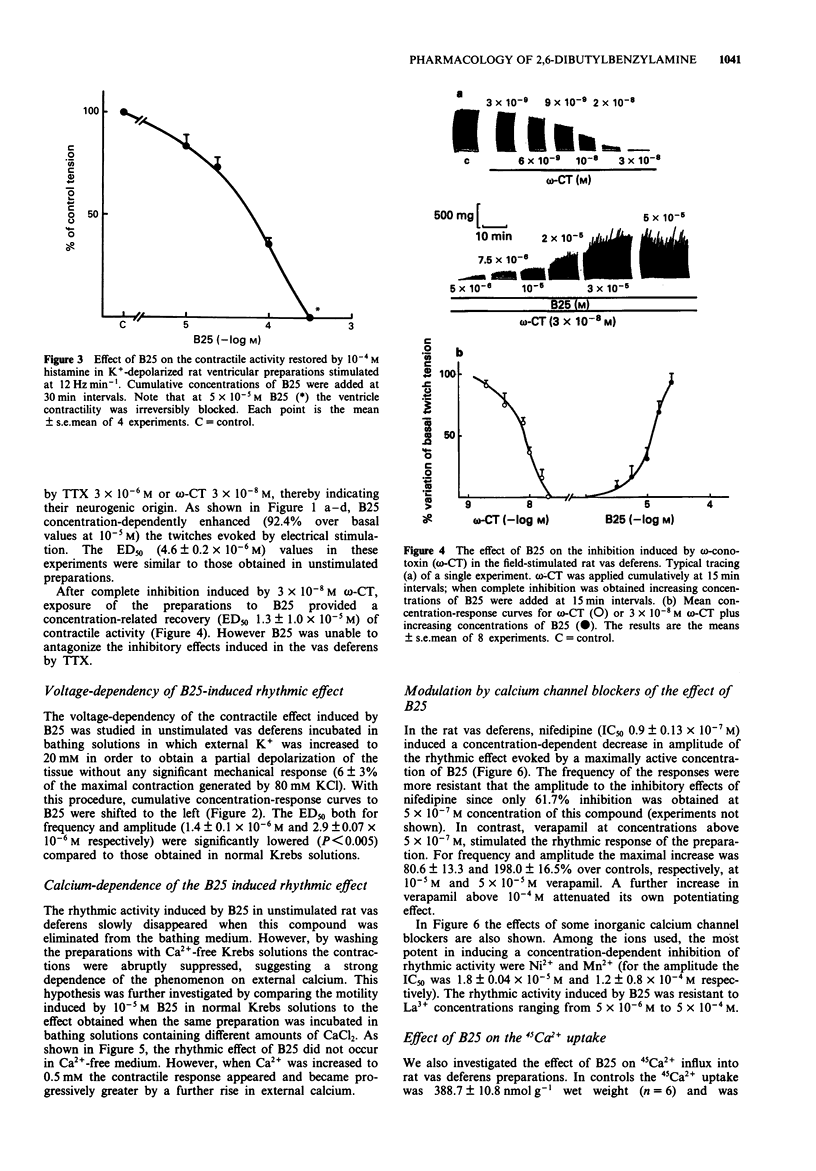
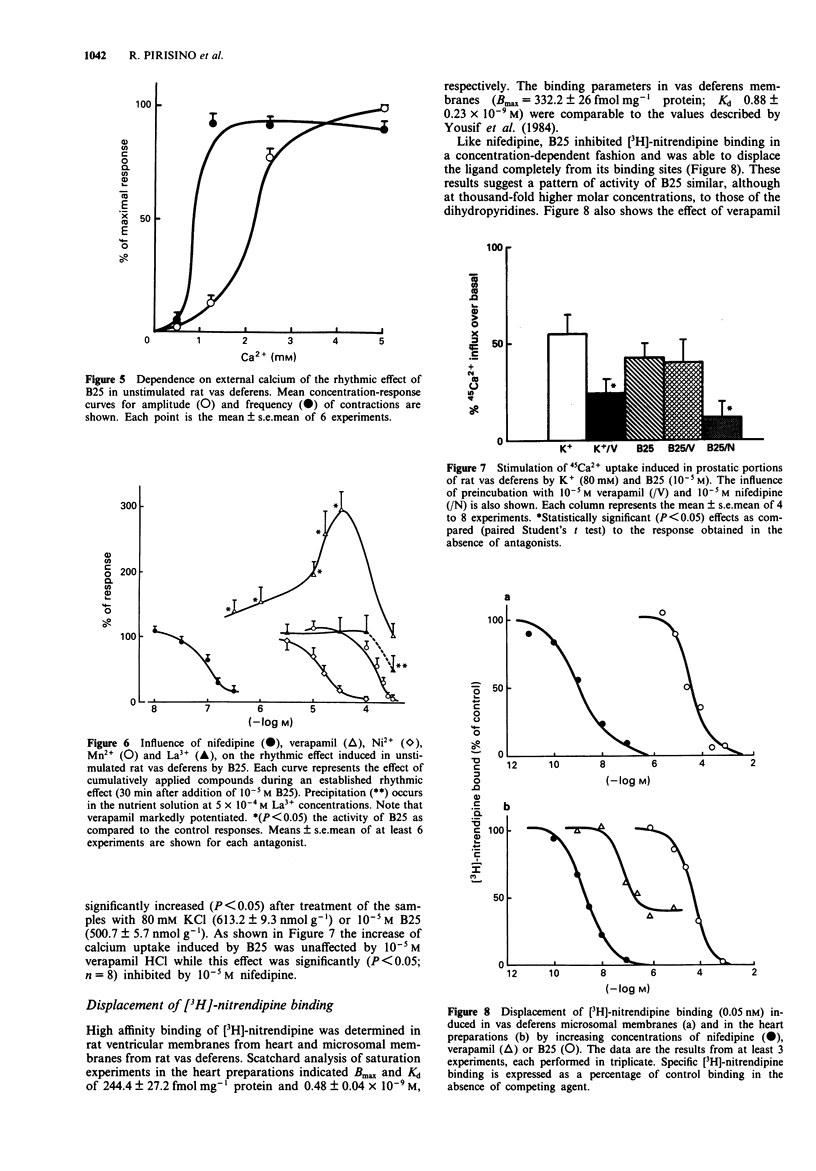
1. In rat isolated vas deferens the new compound 2,6-dibutylbenzylamine (B25) evoked a series of repeating rhythmic contractions. Concentration-response curves constructed for this effect were bell-shaped, indicating a biphasic effect for this compound. By contrast, B25 depressed heart contractility without any visible positive inotropic or chronotropic activity. 2. Experiments with tetrodotoxin, reserpine, capsaicin, alpha-adrenoceptor blocking compounds and other agents permit us to exclude a release of neuromediators or a direct stimulation of post-synaptic receptors to account for the rhythmic effect of B25 in the rat vas deferens. 3. In the same tissue, the increase in 45Ca2+ uptake, the voltage-dependency as well as the dependence of the B25-induced rhythmic activity upon the external calcium concentration indicate a direct activation of voltage-sensitive calcium channels (VSCC). 4. Verapamil paradoxically stimulated the rhythmic effect of B25 in the rat vas deferens. La3+ was inactive while nifedipine was a weak inhibitor. By contrast Ni2+ and Mn2+ ions were good inhibitors (IC50 < 10(-4) M), suggesting that a possible opening of T-type VSCC underlies rhythmic effect of B25. 5. In radioligand binding studies competition experiments with [3H]-nitrendipine indicated that only at high concentrations was B25 able to interact with dihydropyridine-sensitive binding sites of heart and vas deferens smooth muscle. 6. B25 (3-30 microM) counteracted the inhibitory effects of omega-conotoxin GVIA in field-stimulated rat vas deferens.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashoori F., Tomita T. Mechanical response to noradrenaline in calcium-free solution in the rat vas deferens. J Physiol. 1983 May;338:165–178. doi: 10.1113/jphysiol.1983.sp014667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell C., Gillespie J. S., Macrae I. M. Release of noradrenaline and dopamine by nerve stimulation in the guinea-pig and rat vas deferens. Br J Pharmacol. 1984 Mar;81(3):563–569. doi: 10.1111/j.1476-5381.1984.tb10110.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolger G. T., Gengo P., Klockowski R., Luchowski E., Siegel H., Janis R. A., Triggle A. M., Triggle D. J. Characterization of binding of the Ca++ channel antagonist, [3H]nitrendipine, to guinea-pig ileal smooth muscle. J Pharmacol Exp Ther. 1983 May;225(2):291–309. [PubMed] [Google Scholar]
- Brading A. F., Burdyga T. V., Scripnyuk Z. D. The effects of papaverine on the electrical and mechanical activity of the guinea-pig ureter. J Physiol. 1983 Jan;334:79–89. doi: 10.1113/jphysiol.1983.sp014481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown A. M., Birnbaumer L. Direct G protein gating of ion channels. Am J Physiol. 1988 Mar;254(3 Pt 2):H401–H410. doi: 10.1152/ajpheart.1988.254.3.H401. [DOI] [PubMed] [Google Scholar]
- Chang K. J., Triggle D. J. Quantitative aspects of drug-receptor interactions. I. Ca2+ and cholinergic receptor activation in smooth muscle: a basic model for drug-receptor interactions. J Theor Biol. 1973 Jul;40(1):125–154. doi: 10.1016/0022-5193(73)90168-9. [DOI] [PubMed] [Google Scholar]
- DeLean A., Munson P. J., Rodbard D. Simultaneous analysis of families of sigmoidal curves: application to bioassay, radioligand assay, and physiological dose-response curves. Am J Physiol. 1978 Aug;235(2):E97–102. doi: 10.1152/ajpendo.1978.235.2.E97. [DOI] [PubMed] [Google Scholar]
- Dubé G. P., Baik Y. H., Schwartz A. Effects of a novel calcium channel agonist dihydropyridine analogue, Bay k 8644, on pig coronary artery: biphasic mechanical response and paradoxical potentiation of contraction by diltiazem and nimodipine. J Cardiovasc Pharmacol. 1985 Mar-Apr;7(2):377–389. doi: 10.1097/00005344-198503000-00025. [DOI] [PubMed] [Google Scholar]
- Ferrante J., Triggle D. J. Drug- and disease-induced regulation of voltage-dependent calcium channels. Pharmacol Rev. 1990 Mar;42(1):29–44. [PubMed] [Google Scholar]
- French A. M., Scott N. C. Evidence to support the hypothesis that ATP is a co-transmitter in rat vas deferens. Experientia. 1983 Mar 15;39(3):264–266. doi: 10.1007/BF01955295. [DOI] [PubMed] [Google Scholar]
- Hay D. W., Wadsworth R. M. Effect of verapamil on rhythmic contractions in isolated rat vasa deferentia [proceedings]. Br J Pharmacol. 1980 Jan;68(1):182P–183P. [PMC free article] [PubMed] [Google Scholar]
- Hay D. W., Wadsworth R. M. Effects of some organic calcium antagonists and other procedures affecting Ca2+ Translocation on KCl-induced contractions in the rat vas deferens. Br J Pharmacol. 1982 May;76(1):103–113. doi: 10.1111/j.1476-5381.1982.tb09195.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hay D. W., Wadsworth R. M. The effects of calcium channel inhibitors and other procedures affecting calcium translocation on drug-induced rhythmic contractions in the rat vas deferens. Br J Pharmacol. 1983 Jun;79(2):347–362. doi: 10.1111/j.1476-5381.1983.tb11007.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huddart H., Butler D. J. Field stimulation responses of rat urinary bladder detrusor smooth-muscle. Dependence upon slow calcium channel activity determined by K+ depolarization and calcium antagonists. Gen Pharmacol. 1986;17(6):695–703. doi: 10.1016/0306-3623(86)90302-2. [DOI] [PubMed] [Google Scholar]
- Hurwitz L., Suria A. The link between agonist action and response in smooth muscle. Annu Rev Pharmacol. 1971;11:303–326. doi: 10.1146/annurev.pa.11.040171.001511. [DOI] [PubMed] [Google Scholar]
- Keith R. A., Mangano T. J., Pacheco M. A., Salama A. I. Characterization of the effects of omega-conotoxin GVIA on the responses of voltage-sensitive calcium channels. J Auton Pharmacol. 1989 Aug;9(4):243–252. doi: 10.1111/j.1474-8673.1989.tb00215.x. [DOI] [PubMed] [Google Scholar]
- Knoll J., Somogyi G. T., Illés P., Vizi E. S. Acetylcholine release from isolated vas deferens of the rat. Naunyn Schmiedebergs Arch Pharmacol. 1972;274(2):198–202. doi: 10.1007/BF00501855. [DOI] [PubMed] [Google Scholar]
- Langton P. D., Huddart H. The involvement of fast calcium channel activity in the selective activation of phasic contractions by partial depolarization in rat vas deferens smooth muscle. Gen Pharmacol. 1987;18(1):47–55. doi: 10.1016/0306-3623(87)90169-8. [DOI] [PubMed] [Google Scholar]
- Ledda F., Mantelli L., Manzini S., Amerini S., Mugelli A. Electrophysiological and antiarrhythmic properties of propafenon in isolated cardiac preparations. J Cardiovasc Pharmacol. 1981 Nov-Dec;3(6):1162–1173. doi: 10.1097/00005344-198111000-00002. [DOI] [PubMed] [Google Scholar]
- Lee T. J., Westfall D. P., Fleming W. W. The correlation between spontaneous contractions and postjunctional supersensitivity of the smooth muscle of the rat vas deferens. J Pharmacol Exp Ther. 1975 Jan;192(1):136–148. [PubMed] [Google Scholar]
- Maggi C. A., Giuliani S., Santicioli P., Meli A. Capsaicin-induced inhibition of motility of the rat isolated vas deferens: do multiple neuropeptides mediate the visceromotor effects of capsaicin? J Auton Pharmacol. 1987 Sep;7(3):243–255. doi: 10.1111/j.1474-8673.1987.tb00153.x. [DOI] [PubMed] [Google Scholar]
- Mantelli L., Manzini S., Mugelli A., Ledda F. The influence of some cardiodepressant drugs on the histamine-induced restoration of contractility in potassium-depolarized heart preparations. Arch Int Pharmacodyn Ther. 1981 Nov;254(1):99–108. [PubMed] [Google Scholar]
- Matucci R., Ottaviani M. F., Campana S., Bianchi B., Bennardini F., Moneti G., Giotti A., Franconi F. Do ethoxy radicals reduce 3H-nitrendipine binding in rat cardiac membranes? Pharmacol Res. 1992 Sep;26(2):151–159. doi: 10.1016/s1043-6618(05)80128-8. [DOI] [PubMed] [Google Scholar]
- Meisheri K. D., Hwang O., van Breemen C. Evidence for two separated Ca2+ pathways in smooth muscle plasmalemma. J Membr Biol. 1981 Mar 15;59(1):19–25. doi: 10.1007/BF01870817. [DOI] [PubMed] [Google Scholar]
- Miller R. J. Multiple calcium channels and neuronal function. Science. 1987 Jan 2;235(4784):46–52. doi: 10.1126/science.2432656. [DOI] [PubMed] [Google Scholar]
- Mishra S. K., Das P. K., Sanyal A. K. Barium-induced contraction of rat vas deferens in calcium-free solution. Arch Int Pharmacodyn Ther. 1988 Jul-Aug;294:85–98. [PubMed] [Google Scholar]
- Morishita H., Furukawa T. Low calcium and calcium antagonists potentiate the contraction of guinea-pig vas deferens induced by ATP: a permissive role for P2-purinoceptors. Eur J Pharmacol. 1989 May 30;164(3):507–513. doi: 10.1016/0014-2999(89)90258-6. [DOI] [PubMed] [Google Scholar]
- Moritoki H., Iwamoto T., Kanaya J., Maeshiba Y., Ishida Y., Fukuda H. Verapamil enhances the non-adrenergic twitch response of rat vas deferens. Eur J Pharmacol. 1987 Aug 4;140(1):75–83. doi: 10.1016/0014-2999(87)90636-4. [DOI] [PubMed] [Google Scholar]
- Mottram D. R., Wadhwani D. The sympathomimetic activity of fenfluramine hydrochloride on rat vas deferens. Br J Pharmacol. 1977 Apr;59(4):615–620. doi: 10.1111/j.1476-5381.1977.tb07729.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munson P. J., Rodbard D. Ligand: a versatile computerized approach for characterization of ligand-binding systems. Anal Biochem. 1980 Sep 1;107(1):220–239. doi: 10.1016/0003-2697(80)90515-1. [DOI] [PubMed] [Google Scholar]
- Nakazawa K., Inoue K., Fujimori K., Takanaka A. Neurokinin A suppresses a voltage-gated K+ current in smooth muscle cells from rat vas deferens. Eur J Pharmacol. 1990 Jun 21;182(1):189–192. doi: 10.1016/0014-2999(90)90512-5. [DOI] [PubMed] [Google Scholar]
- PATON W. D. The response of the guineapig ileum to electrical stimulation by coaxial electrodes. J Physiol. 1955 Feb 28;127(2):40–1P. [PubMed] [Google Scholar]
- Rae G. A., Calixto J. B. Interactions of calcium antagonists and the calcium channel agonist Bay K 8644 on neurotransmission of the mouse isolated vas deferens. Br J Pharmacol. 1989 Feb;96(2):333–340. doi: 10.1111/j.1476-5381.1989.tb11822.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen H., Barrett P. Q. Calcium messenger system: an integrated view. Physiol Rev. 1984 Jul;64(3):938–984. doi: 10.1152/physrev.1984.64.3.938. [DOI] [PubMed] [Google Scholar]
- Sakai Y., Aihara K., Honda H., Inazu M. Calcium mobilization and phosphatidylinositol turnover in vas deferens smooth muscle of diabetic rats. Eur J Pharmacol. 1989 Mar 29;162(3):475–481. doi: 10.1016/0014-2999(89)90338-5. [DOI] [PubMed] [Google Scholar]
- Sakai Y., Honda H. Hyperactivity of Ca channels in vasa deferentia smooth muscle of diabetic rats. Pharmacol Biochem Behav. 1987 Jun;27(2):227–229. doi: 10.1016/0091-3057(87)90562-4. [DOI] [PubMed] [Google Scholar]
- Schwartz A., Grupp I. L., Grupp G., Williams J. S., Vaghy P. L. Effects of dihydropyridine calcium channel modulators in the heart: pharmacological and radioligand binding correlations. Biochem Biophys Res Commun. 1984 Nov 30;125(1):387–394. doi: 10.1016/s0006-291x(84)80380-0. [DOI] [PubMed] [Google Scholar]
- Scott R. H., Dolphin A. C. Activation of a G protein promotes agonist responses to calcium channel ligands. Nature. 1987 Dec 24;330(6150):760–762. doi: 10.1038/330760a0. [DOI] [PubMed] [Google Scholar]
- Suzuki N., Gomi Y. Effects of calcium-entry modulators on the biphasic neurogenic contractions in the circular muscle of guinea pig vas deferens. Jpn J Pharmacol. 1989 Jan;49(1):59–65. doi: 10.1254/jjp.49.59. [DOI] [PubMed] [Google Scholar]
- Thomas G., Gross R., Schramm M. Calcium channel modulation: ability to inhibit or promote calcium influx resides in the same dihydropyridine molecule. J Cardiovasc Pharmacol. 1984 Nov-Dec;6(6):1170–1176. [PubMed] [Google Scholar]
- Triggle C. R., Swamy V. C., Triggle D. J. Calcium antagonists and contractile responses in rat vas deferens and guinea pig ileal smooth muscle. Can J Physiol Pharmacol. 1979 Aug;57(8):804–818. doi: 10.1139/y79-124. [DOI] [PubMed] [Google Scholar]
- Westfall D. P., McClure D. C., Fleming W. W. The effects of denervation, decentralization and cocaine on the response of the smooth muscle of the guinea-pig vas deferens to various drugs. J Pharmacol Exp Ther. 1972 May;181(2):328–338. [PubMed] [Google Scholar]
- Yousif F. B., Bolger G. T., Ruzycky A., Triggle D. J. Ca2+ channel antagonist actions in bladder smooth muscle: comparative pharmacologic and [3H]nitrendipine binding studies. Can J Physiol Pharmacol. 1985 May;63(5):453–462. doi: 10.1139/y85-079. [DOI] [PubMed] [Google Scholar]


