Abstract
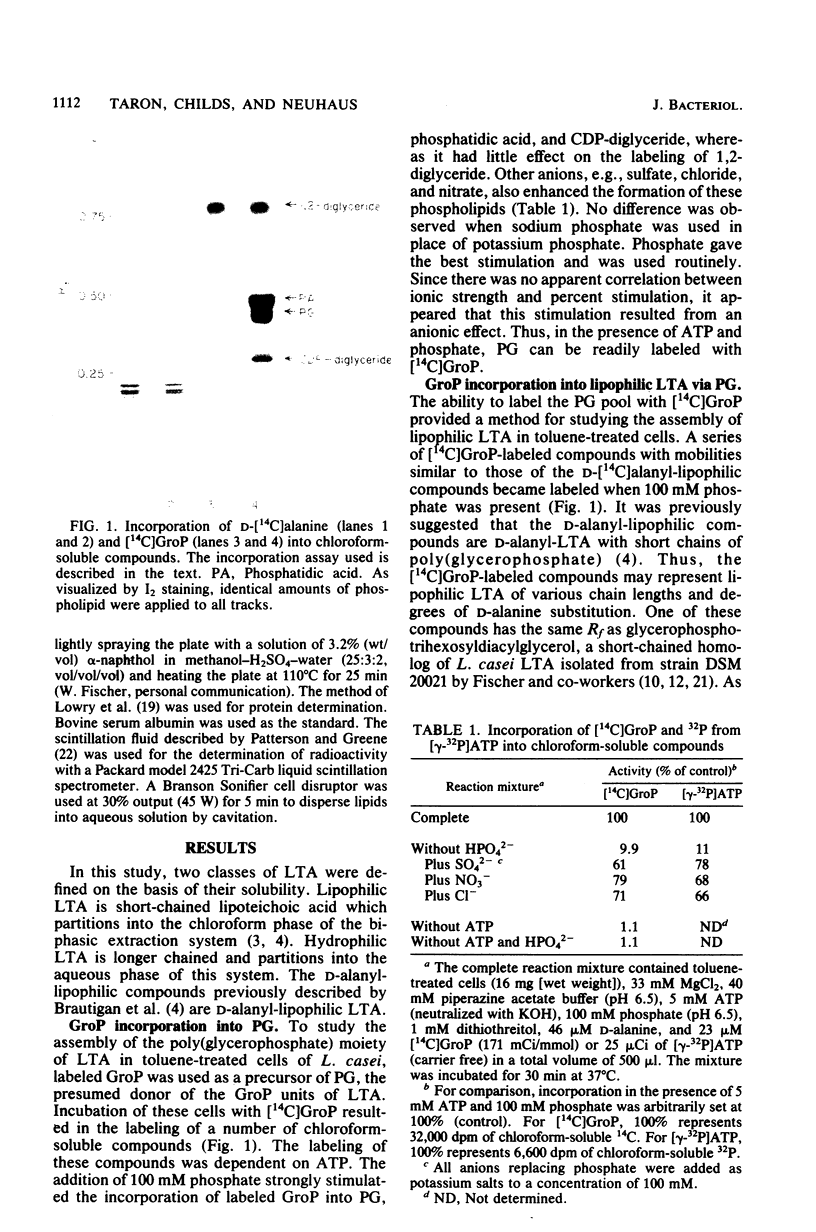
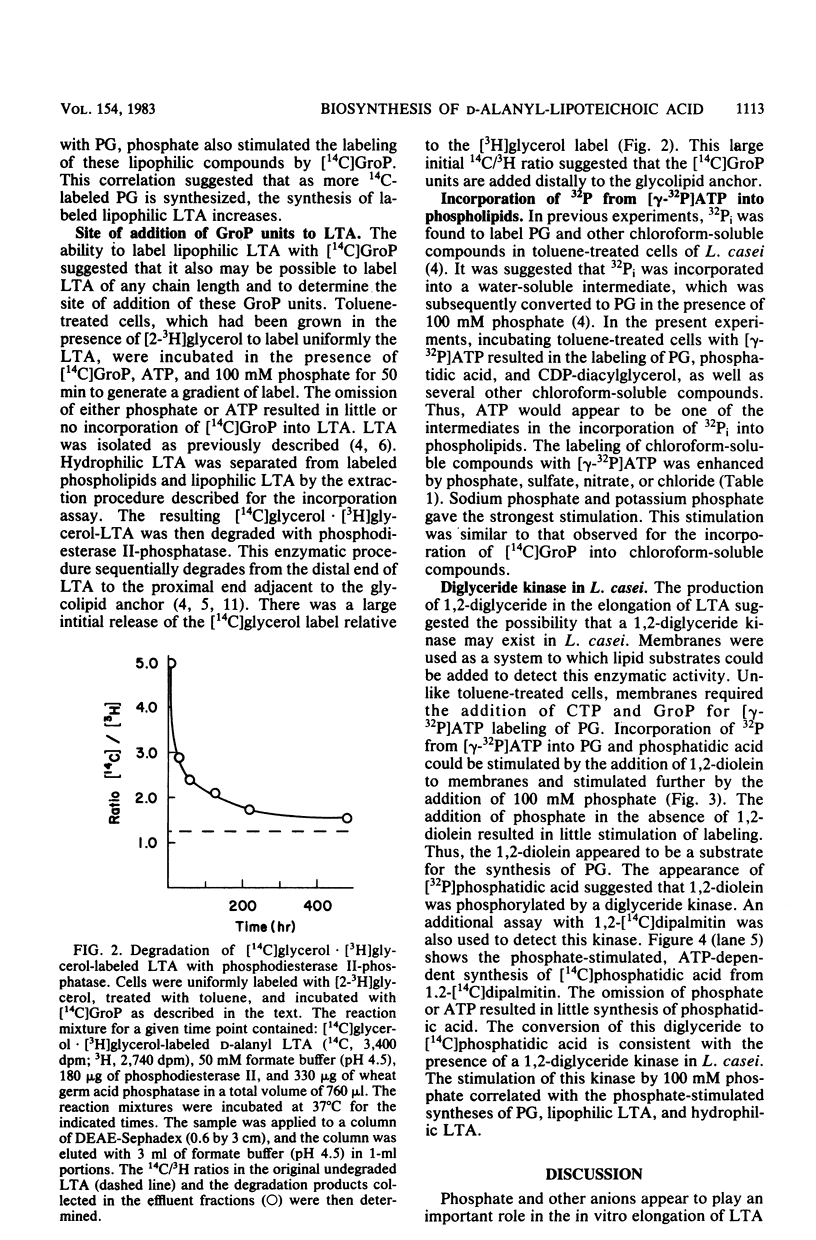
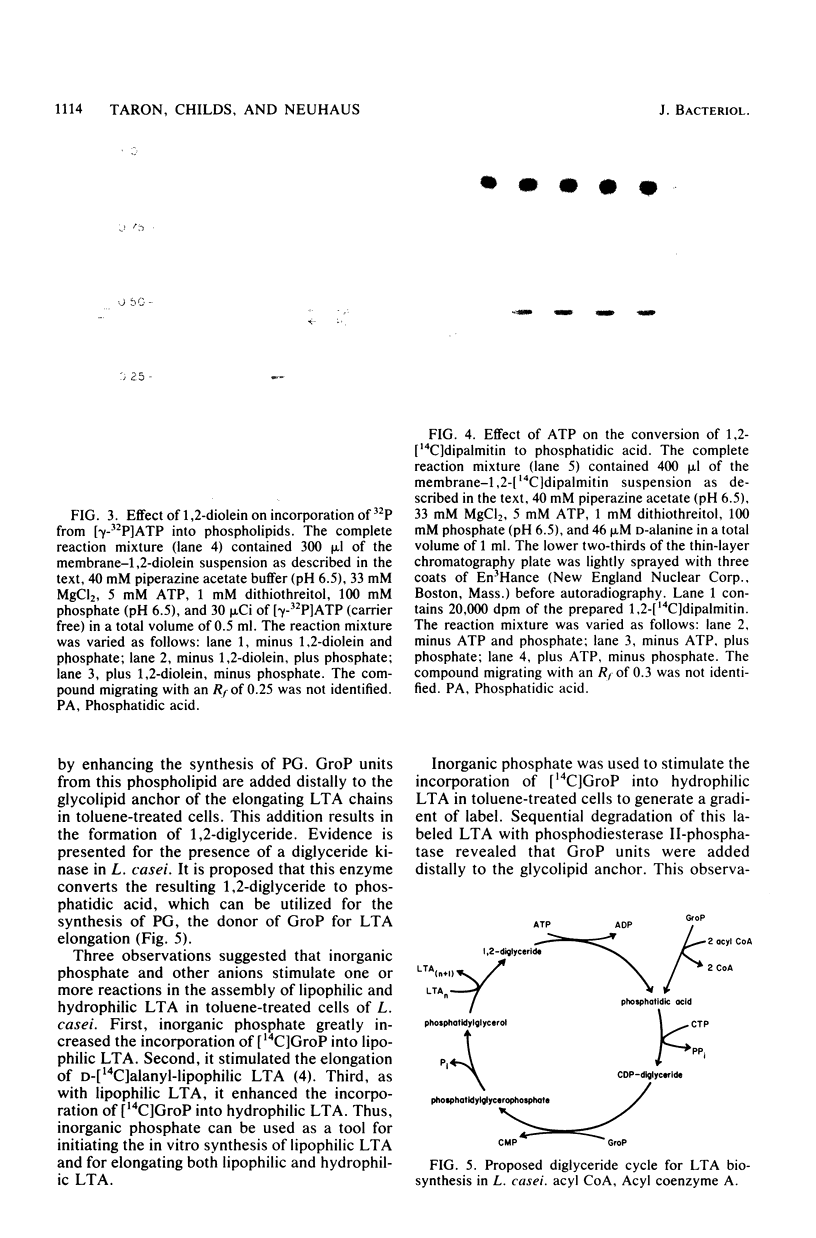
Lipophilic and hydrophilic D-alanyl-lipoteichoic acids are elongated in Lactobacillus casei by the transfer of sn-glycerol 1-phosphate units from phosphatidylglycerol to the poly(glycerophosphate) moiety of the polymer. These sn-glycerol 1-phosphate units are added to the end of the poly(glycerophosphate) which is distal to the glycolipid anchor; 1,2-diglyceride results from this addition. The presence of a diglyceride kinase was suggested by the ATP-dependent phosphorylation of 1,2-diglyceride to phosphatidic acid. Inorganic phosphate was used to initiate the synthesis of lipophilic lipoteichoic acid (LTA) and the elongation of both lipophilic and hydrophilic LTA. Three observations suggest that phosphate and other anions play a role in the in vitro synthesis of LTA and its precursors. First, the conversion of 1,2-diglyceride to phosphatidic acid by diglyceride kinase was stimulated. Second, the synthesis of phosphatidylglycerol was increased. Third, the elongation of lipophilic and hydrophilic LTA was enhanced. These observations indicated that one effect of phosphate might be to enhance the utilization of 1,2-diglyceride for the synthesis of phosphatidic acid. This phospholipid is a precursor of phosphatidylglycerol, the donor of sn-glycerol 1-phosphate for elongation of LTA.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BLIGH E. G., DYER W. J. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959 Aug;37(8):911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Ballesta J. P., De Garcia C. L., Schaechter M. Turnover of phosphatidylglycerol in Escherichia coli. J Bacteriol. 1973 Oct;116(1):210–214. doi: 10.1128/jb.116.1.210-214.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bevers E. M., Singal S. A., Op den Kamp J. A., van Deenen L. L. Recognition of different pools of phosphatidylglycerol in intact cells and isolated membranes of Acholeplasma laidlawii by phospholipase A2. Biochemistry. 1977 Apr 5;16(7):1290–1295. doi: 10.1021/bi00626a008. [DOI] [PubMed] [Google Scholar]
- Brautigan V. M., Childs W. C., 3rd, Neuhaus F. C. Biosynthesis of D-alanyl-lipoteichoic acid in Lactobacillus casei: D-alanyl-lipophilic compounds as intermediates. J Bacteriol. 1981 Apr;146(1):239–250. doi: 10.1128/jb.146.1.239-250.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabacungan E., Pieringer R. A. Mode of elongation of the glycerol phosphate polymer of membrane lipoteichoic acid of Streptococcus faecium ATCC 9790. J Bacteriol. 1981 Jul;147(1):75–79. doi: 10.1128/jb.147.1.75-79.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Childs W. C., 3rd, Neuhaus F. C. Biosynthesis of D-alanyl-lipoteichoic acid: characterization of ester-linked D-alanine in the in vitro-synthesized product. J Bacteriol. 1980 Jul;143(1):293–301. doi: 10.1128/jb.143.1.293-301.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emdur L. I., Chiu T. H. Turnover of phosphatidylglycerol in Streptococcus sanguis. Biochem Biophys Res Commun. 1974 Aug 5;59(3):1137–1144. doi: 10.1016/s0006-291x(74)80097-5. [DOI] [PubMed] [Google Scholar]
- Emdur L., Chiu T. The role of phosphatidylglycerol in the in vitro biosynthesis of teichoic acid and lipoteichoic acid. FEBS Lett. 1975 Jul 15;55(1):216–219. doi: 10.1016/0014-5793(75)80995-1. [DOI] [PubMed] [Google Scholar]
- Fischer W., Koch H. U., Rösel P., Fiedler F. Alanine ester-containing native lipoteichoic acids do not act as lipoteichoic acid carrier. Isolation, structural and functional characterization. J Biol Chem. 1980 May 25;255(10):4557–4562. [PubMed] [Google Scholar]
- Fischer W., Laine R. A., Nakano M. On the relationship between glycerophosphoglycolipids and lipoteichoic acids in Gram-positive bacteria. II. Structures of glycerophosphoglycolipids. Biochim Biophys Acta. 1978 Mar 30;528(3):298–308. doi: 10.1016/0005-2760(78)90019-x. [DOI] [PubMed] [Google Scholar]
- Ganfield M. C., Pieringer R. A. The biosynthesis of nascent membrane lipoteichoic acid of Streptococcus faecium (S. faecalis ATCC 9790) from phosphatidylkojibiosyl diacylglycerol and phosphatidylglycerol. J Biol Chem. 1980 Jun 10;255(11):5164–5169. [PubMed] [Google Scholar]
- Glaser L., Lindsay B. The synthesis of lipoteichoic acid carrier. Biochem Biophys Res Commun. 1974 Aug 5;59(3):1131–1136. doi: 10.1016/s0006-291x(74)80096-3. [DOI] [PubMed] [Google Scholar]
- Kalomiris E., Bardin C., Neuhaus F. C. Biosynthesis of peptidoglycan in Gaffkya homari: reactivation of membranes by freeze-thawing in the presence and absence of walls. J Bacteriol. 1982 May;150(2):535–544. doi: 10.1128/jb.150.2.535-544.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Linzer R., Neuhaus F. C. Biosynthesis of membrane teichoic acid. A role of the D-alanine-activating enzyme. J Biol Chem. 1973 May 10;248(9):3196–3201. [PubMed] [Google Scholar]
- Lombardi F. J., Chen S. L., Fulco A. J. A rapidly metabolizing pool of phosphatidylglycerol as a precursor of phosphatidylethanolamine and diglyceride in Bacillus megaterium. J Bacteriol. 1980 Feb;141(2):626–634. doi: 10.1128/jb.141.2.626-634.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lombardi F. J., Fulco A. J. Two distinct pools of membrane phosphatidylglycerol in Bacillus megaterium. J Bacteriol. 1980 Feb;141(2):618–625. doi: 10.1128/jb.141.2.618-625.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minnikin D. E., Abdolrahimzadeh H., Baddiley J. Variation of polar lipid composition of Bacillus subtilis (Marburg) with different growth conditions. FEBS Lett. 1972 Oct 15;27(1):16–18. doi: 10.1016/0014-5793(72)80398-3. [DOI] [PubMed] [Google Scholar]
- Nakano M., Fischer W. Trihexosyldiacylglycerol and acyltrihexosyldiacylglycerol as lipid anchors of the lipoteichoic acid of Lactobacillus casei DSM 20021. Hoppe Seylers Z Physiol Chem. 1978 Jan;359(1):1–11. doi: 10.1515/bchm.1978.359.1.1. [DOI] [PubMed] [Google Scholar]
- PATTERSON M. S., GREENE R. C. MEASUREMENT OF LOW ENERGY BETA-EMITTERS IN AQUEOUS SOLUTION BY LIQUID SCINTILLATION COUNTING OF EMULSIONS. Anal Chem. 1965 Jun;37:854–857. doi: 10.1021/ac60226a017. [DOI] [PubMed] [Google Scholar]
- PIERINGER R. A., KUNNES R. S. THE BIOSYNTHESIS OF PHOSPHATIDIC ACID AND LYSOPHOSPHATIDIC ACID BY GLYCERIDE PHOSPHOKINASE PATHWAYS IN ESCHERICHIA COLI. J Biol Chem. 1965 Jul;240:2833–2838. [PubMed] [Google Scholar]
- Patterson P. H., Lennarz W. J. Studies on the membranes of bacilli. I. Phospholipid biosynthesis. J Biol Chem. 1971 Feb 25;246(4):1062–1072. [PubMed] [Google Scholar]
- Raetz C. R. Enzymology, genetics, and regulation of membrane phospholipid synthesis in Escherichia coli. Microbiol Rev. 1978 Sep;42(3):614–659. doi: 10.1128/mr.42.3.614-659.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raetz C. R., Newman K. F. Neutral lipid accumulation in the membranes of Escherichia coli mutants lacking diglyceride kinase. J Biol Chem. 1978 Jun 10;253(11):3882–3887. [PubMed] [Google Scholar]
- Reusch V. M., Jr, Neuhaus F. C. D-Alanine: membrane acceptor ligase from Lactobacillus casei. J Biol Chem. 1971 Oct 25;246(20):6136–6143. [PubMed] [Google Scholar]
- Schnebli H. P., Abrams A. Membrane adenosine triphosphatase from Streptococcus faecalis. Preparation and homogeneity. J Biol Chem. 1970 Mar 10;245(5):1115–1121. [PubMed] [Google Scholar]
- Schneider E. G., Kennedy E. P. Partial purification and properties of diglyceride kinase from Escherichia coli. Biochim Biophys Acta. 1976 Aug 23;441(2):201–212. doi: 10.1016/0005-2760(76)90163-6. [DOI] [PubMed] [Google Scholar]
- Schneider E. G., Kennedy E. P. Phosphorylation of ceramide by diglyceride kinase preparations from Escherichia coli. J Biol Chem. 1973 May 25;248(10):3739–3741. [PubMed] [Google Scholar]
- Schneider J. E., Kennedy E. P. A novel phosphodiesterase from Aspergillus niger and its application to the study of membrane-derived oligosaccharides and other glycerol-containing biopolymers. J Biol Chem. 1978 Nov 10;253(21):7738–7743. [PubMed] [Google Scholar]
- Ward J. B. Teichoic and teichuronic acids: biosynthesis, assembly, and location. Microbiol Rev. 1981 Jun;45(2):211–243. doi: 10.1128/mr.45.2.211-243.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]