Abstract
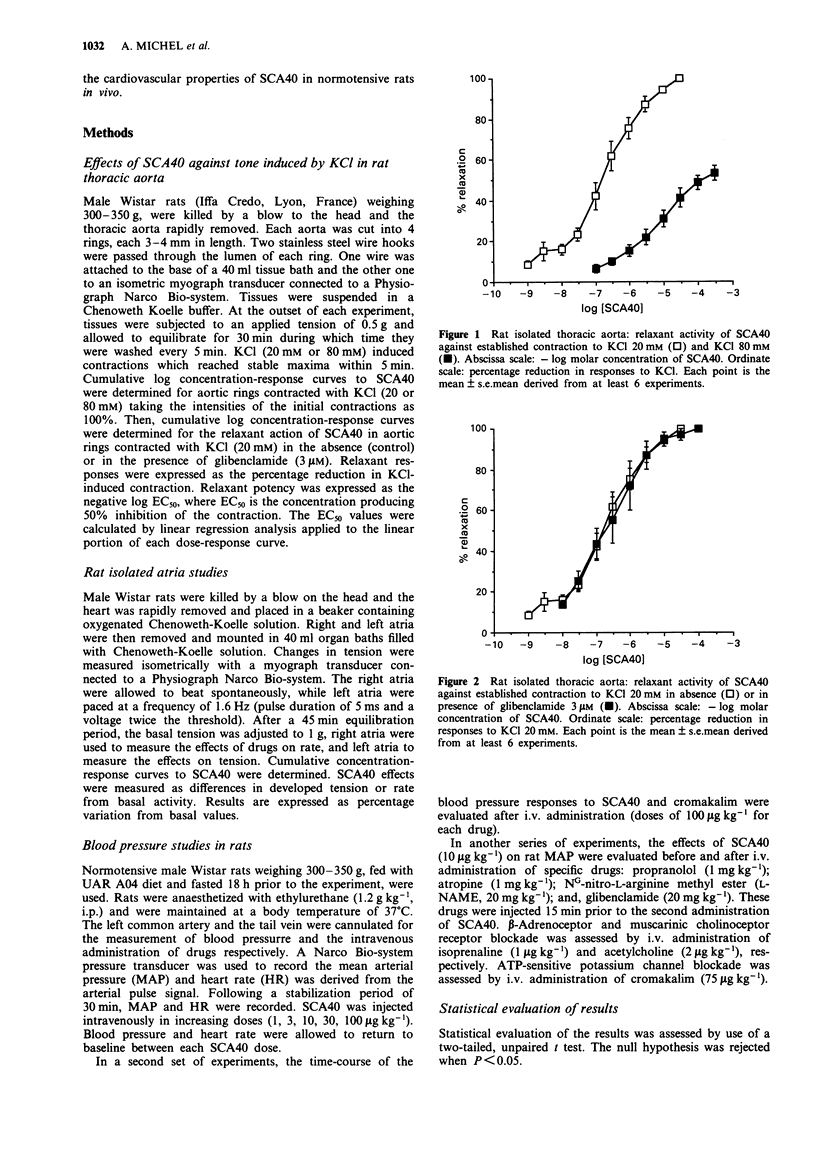
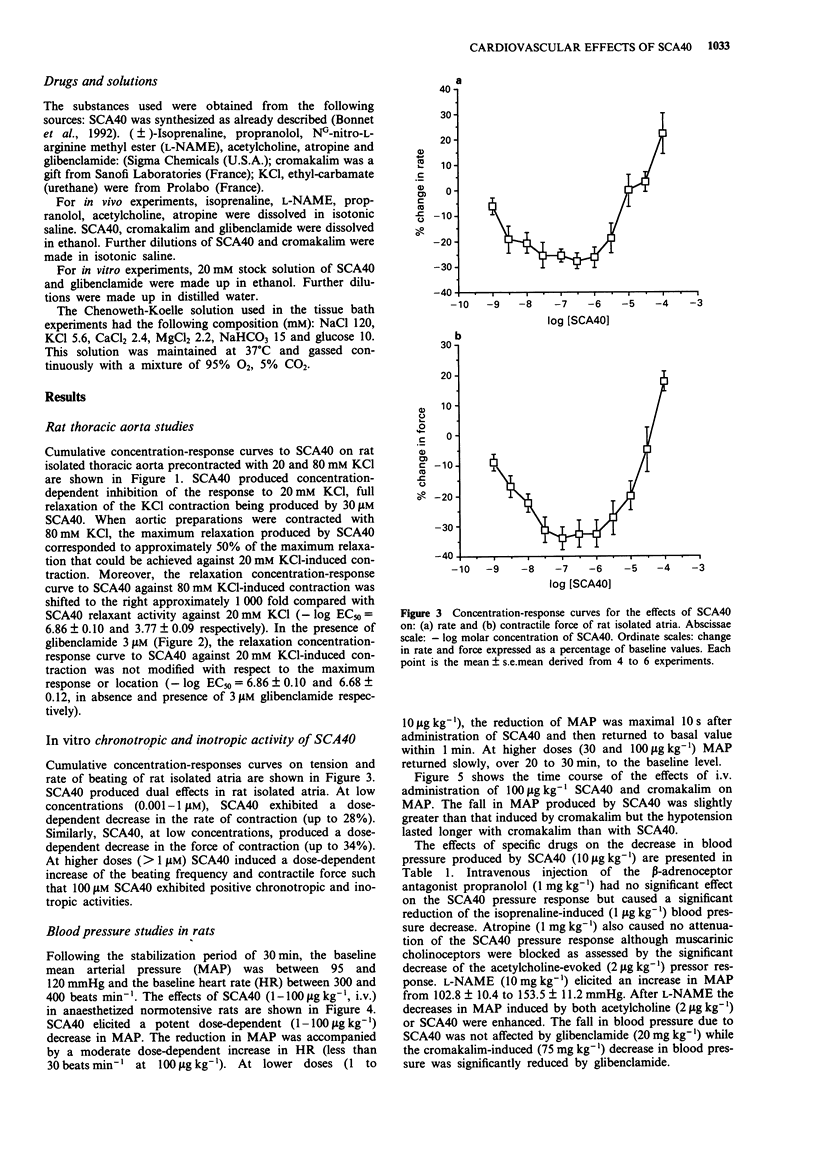
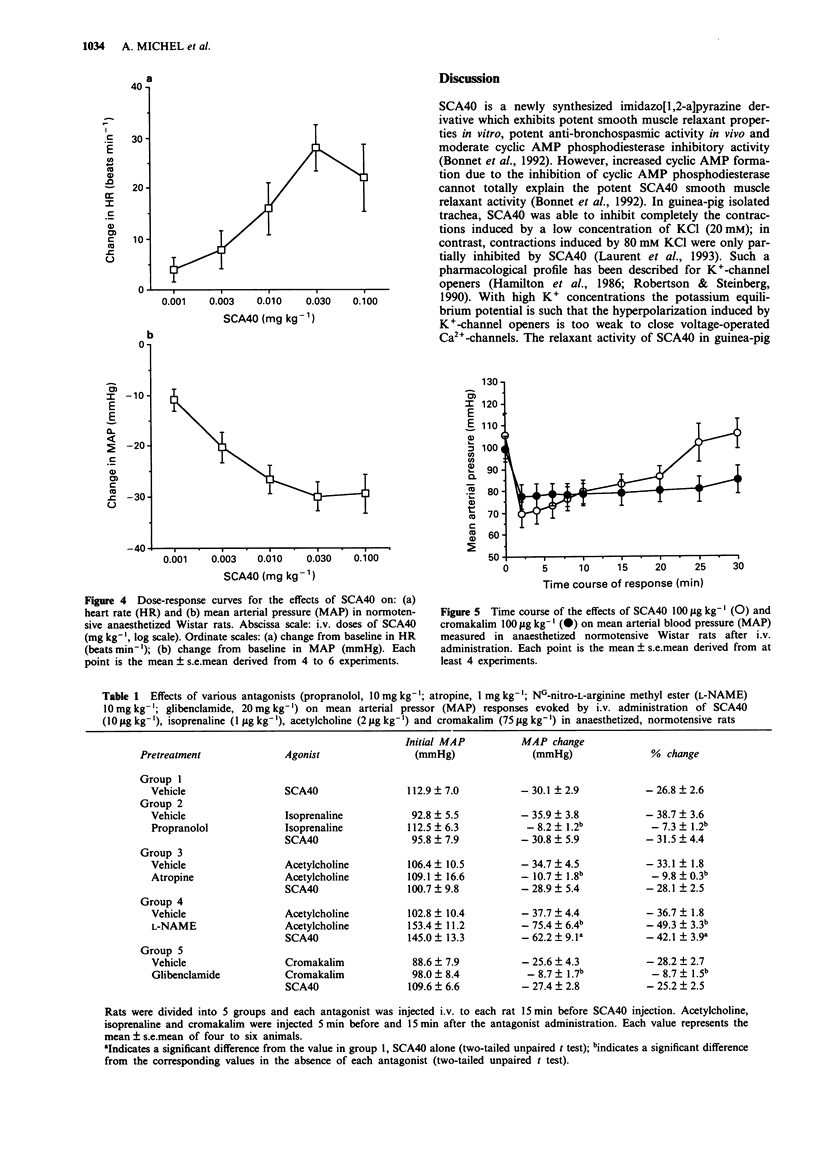
1. Experiments have been performed to investigate the cardiovascular actions in the rat of SCA40, a novel potassium channel opener which is a potent relaxant of guinea-pig airway smooth muscle in vivo and in vitro. 2. SCA40 (0.01-30 microM) caused a complete and concentration-dependent relaxation of rat isolated thoracic aorta contracted with 20 mM KCl but failed to inhibit completely the spasmogenic effects of 80 mM KCl. 3. The ATP-sensitive K(+)-channel blocker, glibenclamide (3 microM), failed to antagonize the relaxant action of SCA40 on 20 mM KCl-contracted rat isolated thoracic aorta. 4. SCA40 (0.001-100 microM) had dual effects on rat isolated atria. At low concentrations, SCA40 produced a concentration-dependent decrease in the rate and force of contractions. At higher concentrations (greater than 1 microM) SCA40 induced concentration-dependent increases of atrial rate and force. 5. In vivo, in normotensive Wistar rats, SCA40 elicited a dose-dependent (1-100 micrograms kg-1) decrease in mean arterial pressure which was accompanied by a moderate dose-dependent increase in heart rate. SCA40 (100 micrograms kg-1) had a slightly greater hypotensive effect than cromakalim (100 micrograms kg-1) but the duration of the hypotension was longer with cromakalim than with SCA40. 6. The hypotensive effect of SCA40 was not reduced by propranolol, atropine, NG-nitro-L-arginine methyl ester (L-NAME) or glibenclamide. 7. It is concluded that the mechanism by with SCA40 relaxes vascular smooth muscle in vitro and in vivo involves activation of K(+)-channels distinct from glibenclamide-sensitive ATP-sensitive K(+)-channels.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Asano M., Masuzawa-Ito K., Matsuda T. Charybdotoxin-sensitive K+ channels regulate the myogenic tone in the resting state of arteries from spontaneously hypertensive rats. Br J Pharmacol. 1993 Jan;108(1):214–222. doi: 10.1111/j.1476-5381.1993.tb13465.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benham C. D., Bolton T. B., Lang R. J., Takewaki T. Calcium-activated potassium channels in single smooth muscle cells of rabbit jejunum and guinea-pig mesenteric artery. J Physiol. 1986 Feb;371:45–67. doi: 10.1113/jphysiol.1986.sp015961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonnet P. A., Michel A., Laurent F., Sablayrolles C., Rechencq E., Mani J. C., Boucard M., Chapat J. P. Synthesis and antibronchospastic activity of 8-alkoxy- and 8-(alkylamino)imidazo[1,2-a]pyrazines. J Med Chem. 1992 Sep 4;35(18):3353–3358. doi: 10.1021/jm00096a008. [DOI] [PubMed] [Google Scholar]
- Brayden J. E., Nelson M. T. Regulation of arterial tone by activation of calcium-dependent potassium channels. Science. 1992 Apr 24;256(5056):532–535. doi: 10.1126/science.1373909. [DOI] [PubMed] [Google Scholar]
- Castle N. A., Haylett D. G., Jenkinson D. H. Toxins in the characterization of potassium channels. Trends Neurosci. 1989 Feb;12(2):59–65. doi: 10.1016/0166-2236(89)90137-9. [DOI] [PubMed] [Google Scholar]
- Cook N. S., Hof R. P. Cardiovascular effects of apamin and BRL 34915 in rats and rabbits. Br J Pharmacol. 1988 Jan;93(1):121–131. doi: 10.1111/j.1476-5381.1988.tb11412.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton T. C., Weir S. W., Weston A. H. Comparison of the effects of BRL 34915 and verapamil on electrical and mechanical activity in rat portal vein. Br J Pharmacol. 1986 May;88(1):103–111. doi: 10.1111/j.1476-5381.1986.tb09476.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue R., Okabe K., Kitamura K., Kuriyama H. A newly identified Ca2+ dependent K+ channel in the smooth muscle membrane of single cells dispersed from the rabbit portal vein. Pflugers Arch. 1986 Feb;406(2):138–143. doi: 10.1007/BF00586674. [DOI] [PubMed] [Google Scholar]
- Laurent F., Michel A., Bonnet P. A., Chapat J. P., Boucard M. Evaluation of the relaxant effects of SCA40, a novel charybdotoxin-sensitive potassium channel opener, in guinea-pig isolated trachealis. Br J Pharmacol. 1993 Mar;108(3):622–626. doi: 10.1111/j.1476-5381.1993.tb12851.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcel van Gelderen E., Heiligers J. P., Saxena P. R. Haemodynamic changes and acetylcholine-induced hypotensive responses after NG-nitro-L-arginine methyl ester in rats and cats. Br J Pharmacol. 1991 Aug;103(4):1899–1904. doi: 10.1111/j.1476-5381.1991.tb12349.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakami M., Furukawa Y., Karasawa Y., Ren L. M., Takayama S., Chiba S. Inhibition by glibenclamide of negative chronotropic and inotropic responses to pinacidil, acetylcholine, and adenosine in the isolated dog heart. J Cardiovasc Pharmacol. 1992 Apr;19(4):618–624. doi: 10.1097/00005344-199204000-00020. [DOI] [PubMed] [Google Scholar]
- Paciorek P. M., Burden D. T., Burke Y. M., Cowlrick I. S., Perkins R. S., Taylor J. C., Waterfall J. F. Preclinical pharmacology of Ro 31-6930, a new potassium channel opener. J Cardiovasc Pharmacol. 1990 Feb;15(2):188–197. doi: 10.1097/00005344-199002000-00003. [DOI] [PubMed] [Google Scholar]
- Quast U., Cook N. S. Moving together: K+ channel openers and ATP-sensitive K+ channels. Trends Pharmacol Sci. 1989 Nov;10(11):431–435. doi: 10.1016/S0165-6147(89)80003-3. [DOI] [PubMed] [Google Scholar]
- Richer C., Pratz J., Mulder P., Mondot S., Giudicelli J. F., Cavero I. Cardiovascular and biological effects of K+ channel openers, a class of drugs with vasorelaxant and cardioprotective properties. Life Sci. 1990;47(19):1693–1705. doi: 10.1016/0024-3205(90)90342-o. [DOI] [PubMed] [Google Scholar]
- Robertson D. W., Steinberg M. I. Potassium channel modulators: scientific applications and therapeutic promise. J Med Chem. 1990 Jun;33(6):1529–1541. doi: 10.1021/jm00168a001. [DOI] [PubMed] [Google Scholar]
- Rusch N. J., De Lucena R. G., Wooldridge T. A., England S. K., Cowley A. W., Jr A Ca(2+)-dependent K+ current is enhanced in arterial membranes of hypertensive rats. Hypertension. 1992 Apr;19(4):301–307. doi: 10.1161/01.hyp.19.4.301. [DOI] [PubMed] [Google Scholar]
- Shigenobu K., Kageyama C., Watanabe M. Action potential shortening and negative inotropic effects of a novel potassium channel opener, NIP-121, as compared with cromakalim in guinea pig ventricular myocardium. Jpn J Pharmacol. 1991 Sep;57(1):117–121. doi: 10.1254/jjp.57.117. [DOI] [PubMed] [Google Scholar]
- Van Renterghem C., Lazdunski M. A small-conductance charybdotoxin-sensitive, apamin-resistant Ca(2+)-activated K+ channel in aortic smooth muscle cells (A7r5 line and primary culture). Pflugers Arch. 1992 Apr;420(5-6):417–423. doi: 10.1007/BF00374614. [DOI] [PubMed] [Google Scholar]
- Vázquez J., Feigenbaum P., Katz G., King V. F., Reuben J. P., Roy-Contancin L., Slaughter R. S., Kaczorowski G. J., Garcia M. L. Characterization of high affinity binding sites for charybdotoxin in sarcolemmal membranes from bovine aortic smooth muscle. Evidence for a direct association with the high conductance calcium-activated potassium channel. J Biol Chem. 1989 Dec 15;264(35):20902–20909. [PubMed] [Google Scholar]
- Yanagisawa T., Hashimoto H., Taira N. Interaction of potassium channel openers and blockers in canine atrial muscle. Br J Pharmacol. 1989 Jul;97(3):753–762. doi: 10.1111/j.1476-5381.1989.tb12013.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanagisawa T., Hashimoto H., Taira N. The negative inotropic effect of nicorandil is independent of cyclic GMP changes: a comparison with pinacidil and cromakalim in canine atrial muscle. Br J Pharmacol. 1988 Oct;95(2):393–398. doi: 10.1111/j.1476-5381.1988.tb11658.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


