Abstract
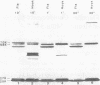
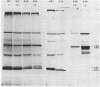
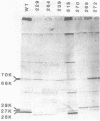
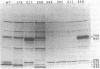
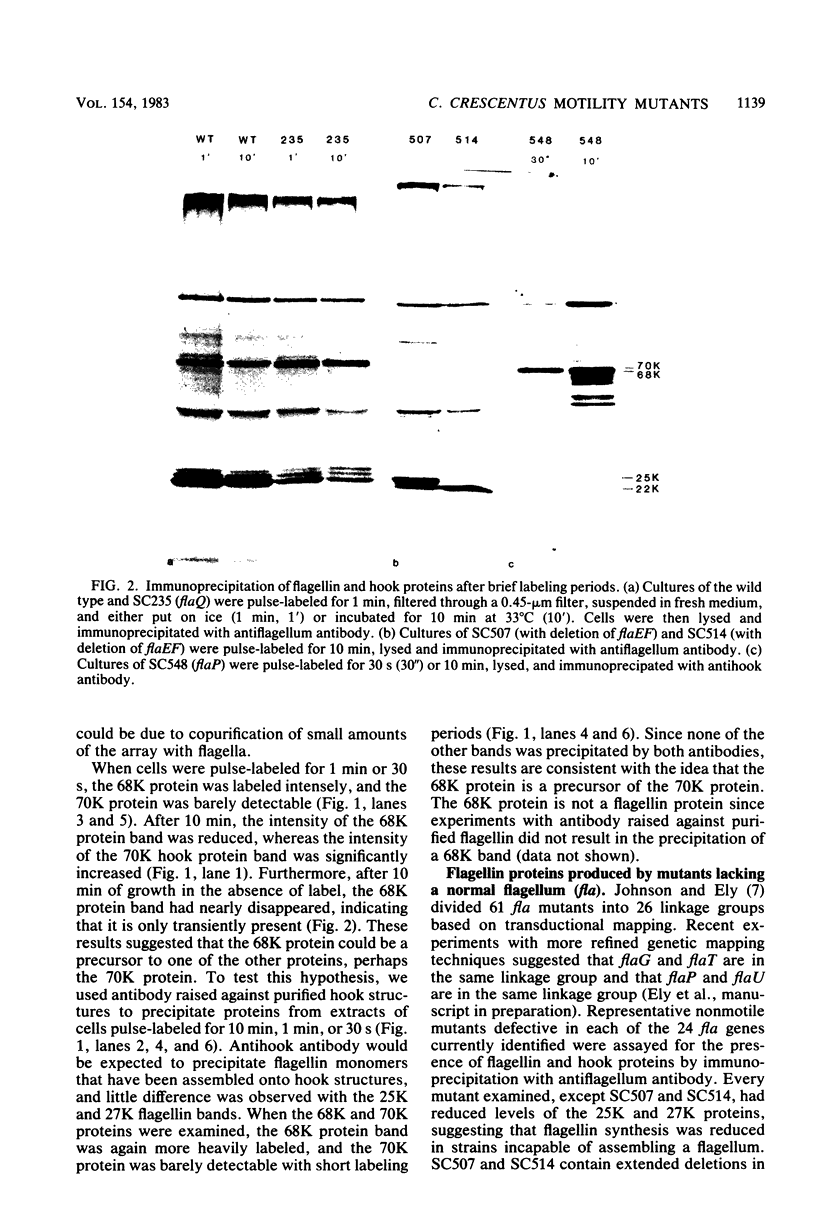
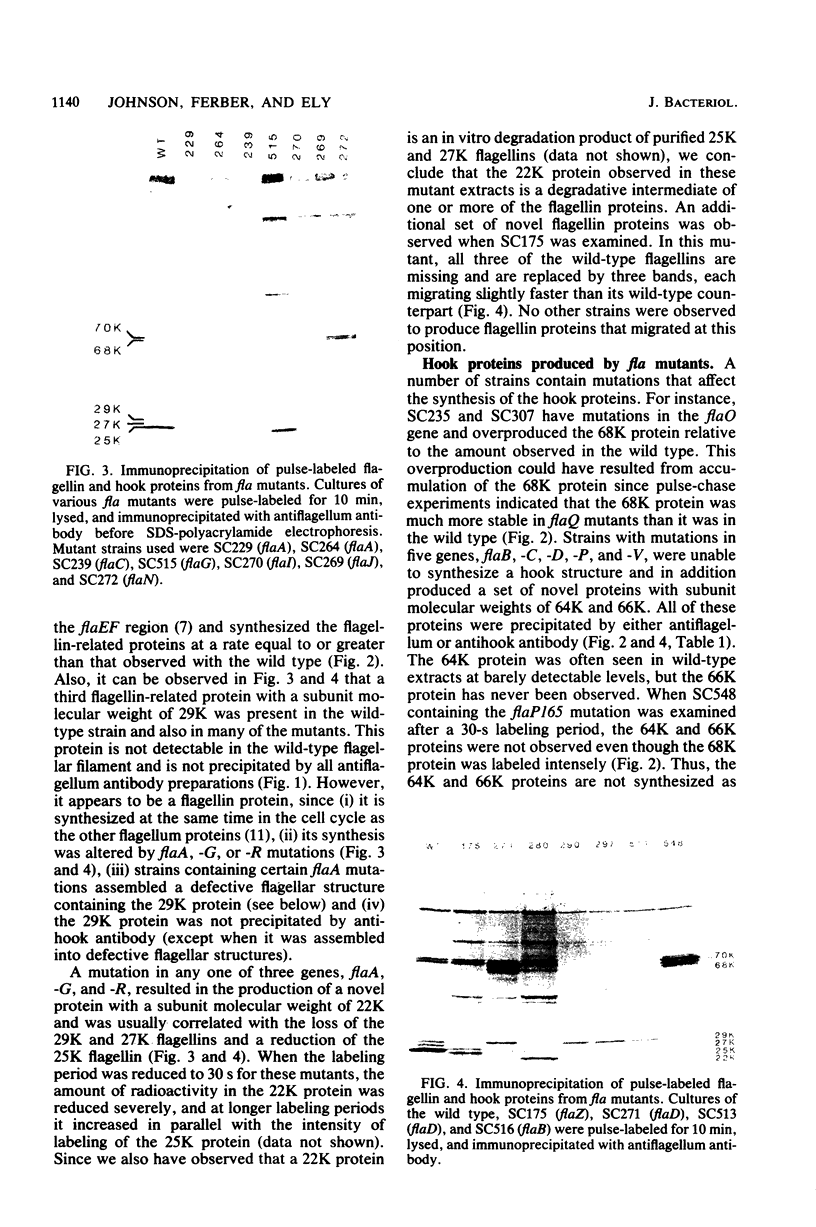
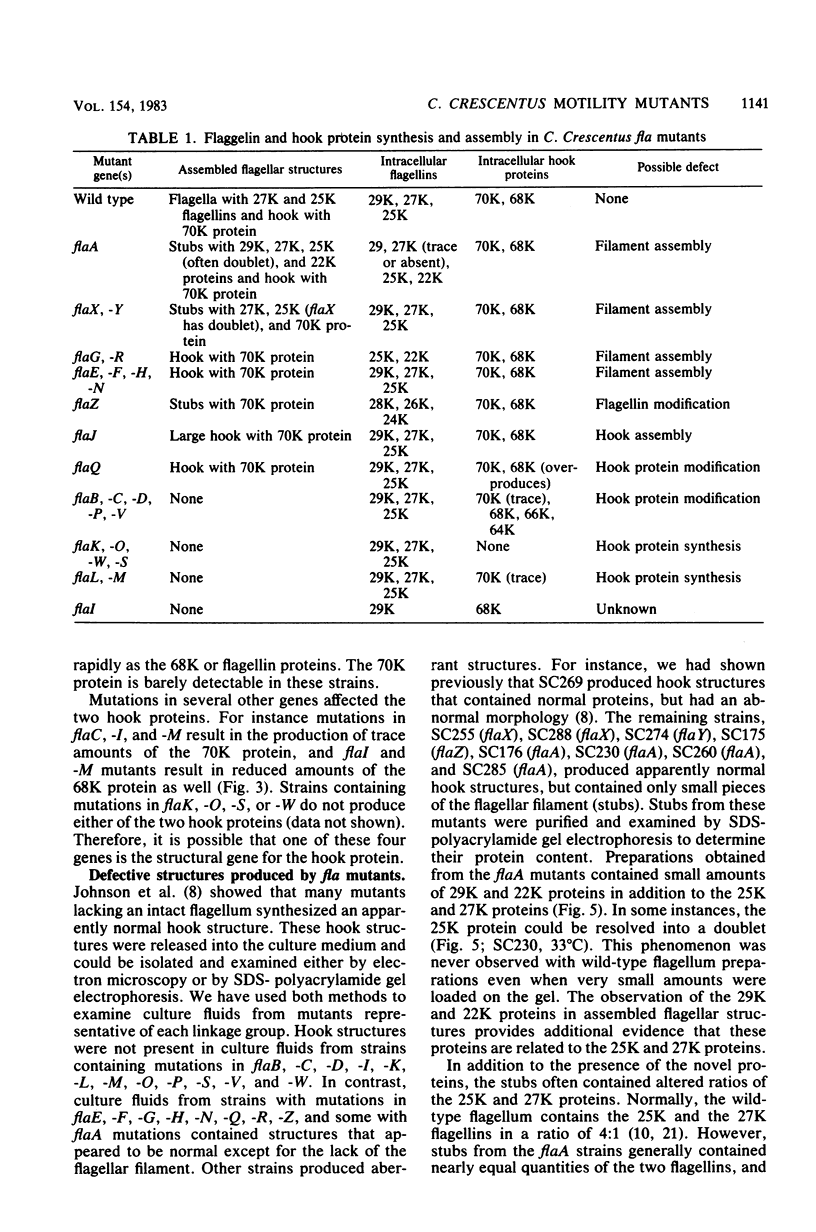
Cultures of wild-type Caulobacter crescentus and strains with fla mutations representing 24 genes were pulse-labeled with 14C-amino acids and analyzed by immunoprecipitation to study the synthesis of flagellar components. Most fla mutants synthesize flagellin proteins at a reduced rate, suggesting the existence of some mechanism to prevent the accumulation of unpolymerized flagellin subunits. Two strains contain deletions that appear to remove a region necessary for this regulation. The hook protein does not seem to be subject to this type of regulation and, in addition, appears to be synthesized as a faster-sedimenting precursor. Mutations in a number of genes result in the appearance of degradation products of either the flagellin or the hook proteins. Mutations in flaA, -X, -Y, or -Z result in the production of filaments (stubs) that contain altered ratios of the flagellin proteins. In some flaA mutants, other flagellin-related proteins were assembled into the stub structures in addition to the flagellins normally present. Taken together, these analyses have begun to provide insight into the roles of individual fla genes in flagellum biogenesis in C. crescentus.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amemiya K., Wu C. W., Shapiro L. Caulobacter crescentus RNA polymerase. Purification and characterization of holoenzyme and core polymerase. J Biol Chem. 1977 Jun 25;252(12):4157–4165. [PubMed] [Google Scholar]
- Fukuda A., Asada M., Koyasu S., Yoshida H., Yaginuma K., Okada Y. Regulation of polar morphogenesis in Caulobacter crescentus. J Bacteriol. 1981 Jan;145(1):559–572. doi: 10.1128/jb.145.1.559-572.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuda A., Koyasu S., Okada Y. Characterization of two flagella-related proteins from Caulobacter crescentus. FEBS Lett. 1978 Nov 1;95(1):70–75. doi: 10.1016/0014-5793(78)80054-4. [DOI] [PubMed] [Google Scholar]
- Gill P. R., Agabian N. A comparative structural analysis of the flagellin monomers of Caulobacter crescentus indicates that these proteins are encoded by two genes. J Bacteriol. 1982 May;150(2):925–933. doi: 10.1128/jb.150.2.925-933.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iino T. Genetics of structure and function of bacterial flagella. Annu Rev Genet. 1977;11:161–182. doi: 10.1146/annurev.ge.11.120177.001113. [DOI] [PubMed] [Google Scholar]
- Johnson R. C., Ely B. Analysis of nonmotile mutants of the dimorphic bacterium Caulobacter crescentus. J Bacteriol. 1979 Jan;137(1):627–634. doi: 10.1128/jb.137.1.627-634.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson R. C., Ely B. Isolation of spontaneously derived mutants of Caulobacter crescentus. Genetics. 1977 May;86(1):25–32. doi: 10.1093/genetics/86.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson R. C., Walsh M. P., Ely B., Shapiro L. Flagellar hook and basal complex of Caulobacter crescentus. J Bacteriol. 1979 Jun;138(3):984–989. doi: 10.1128/jb.138.3.984-989.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lagenaur C., Agabian N. Caulobacter flagellar organelle: synthesis, compartmentation, and assembly. J Bacteriol. 1978 Sep;135(3):1062–1069. doi: 10.1128/jb.135.3.1062-1069.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagenaur C., Agabian N. Physical characterization of Caulobacter crescentus flagella. J Bacteriol. 1976 Oct;128(1):435–444. doi: 10.1128/jb.128.1.435-444.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagenaur C., DeMartini M., Agabian N. Isolation and characterization of Caulobacter crescentus flagellar hooks. J Bacteriol. 1978 Nov;136(2):795–798. doi: 10.1128/jb.136.2.795-798.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milhausen M., Gill P. R., Parker G., Agabian N. Cloning of developmentally regulated flagellin genes from Caulobacter crescentus via immunoprecipitation of polyribosomes. Proc Natl Acad Sci U S A. 1982 Nov;79(22):6847–6851. doi: 10.1073/pnas.79.22.6847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osley M. A., Newton A. Temporal control of the cell cycle in Caulobacter crescentus: roles of DNA chain elongation and completion. J Mol Biol. 1980 Mar 25;138(1):109–128. doi: 10.1016/s0022-2836(80)80007-6. [DOI] [PubMed] [Google Scholar]
- Osley M. A., Sheffery M., Newton A. Regulation of flagellin synthesis in the cell cycle of caulobacter: dependence on DNA replication. Cell. 1977 Oct;12(2):393–400. doi: 10.1016/0092-8674(77)90115-5. [DOI] [PubMed] [Google Scholar]
- Ostrowski M. C., Kistler M. K., Kistler W. S. Effect of castration on the synthesis of seminal vesicle secretory protein IV in the rat. Biochemistry. 1982 Jul 20;21(15):3525–3529. doi: 10.1021/bi00258a001. [DOI] [PubMed] [Google Scholar]
- POINDEXTER J. S. BIOLOGICAL PROPERTIES AND CLASSIFICATION OF THE CAULOBACTER GROUP. Bacteriol Rev. 1964 Sep;28:231–295. doi: 10.1128/br.28.3.231-295.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro L. Differentiation in the Caulobacter cell cycle. Annu Rev Microbiol. 1976;30:377–407. doi: 10.1146/annurev.mi.30.100176.002113. [DOI] [PubMed] [Google Scholar]
- Shapiro L., Maizel J. V., Jr Synthesis and structure of Caulobacter crescentus flagella. J Bacteriol. 1973 Jan;113(1):478–485. doi: 10.1128/jb.113.1.478-485.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheffery M., Newton A. Purification and characterization of a polyhook protein from Caulobacter crescentus. J Bacteriol. 1979 May;138(2):575–583. doi: 10.1128/jb.138.2.575-583.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheffery M., Newton A. Reconstitution and purification of flagellar filaments from Caulobacter crescentus. J Bacteriol. 1977 Dec;132(3):1027–1030. doi: 10.1128/jb.132.3.1027-1030.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheffery M., Newton A. Regulation of periodic protein synthesis in the cell cycle: control of initiation and termination of flagellar gene expression. Cell. 1981 Apr;24(1):49–57. doi: 10.1016/0092-8674(81)90500-6. [DOI] [PubMed] [Google Scholar]
- Silverman M., Simon M. Characterization of Escherichia coli flagellar mutants that are insensitive to catabolite repression. J Bacteriol. 1974 Dec;120(3):1196–1203. doi: 10.1128/jb.120.3.1196-1203.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smit J., Grano D. A., Glaeser R. M., Agabian N. Periodic surface array in Caulobacter crescentus: fine structure and chemical analysis. J Bacteriol. 1981 Jun;146(3):1135–1150. doi: 10.1128/jb.146.3.1135-1150.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki H., Iino T. Absence of messenger ribonucleic acid specific for flagellin in non-flagellate mutants of Salmonella. J Mol Biol. 1975 Jul 15;95(4):549–556. doi: 10.1016/0022-2836(75)90316-2. [DOI] [PubMed] [Google Scholar]
- Weissborn A., Steinmann H. M., Shapiro L. Characterization of the proteins of the Caulobacter crescentus flagellar filament. Peptide analysis and filament organization. J Biol Chem. 1982 Feb 25;257(4):2066–2074. [PubMed] [Google Scholar]