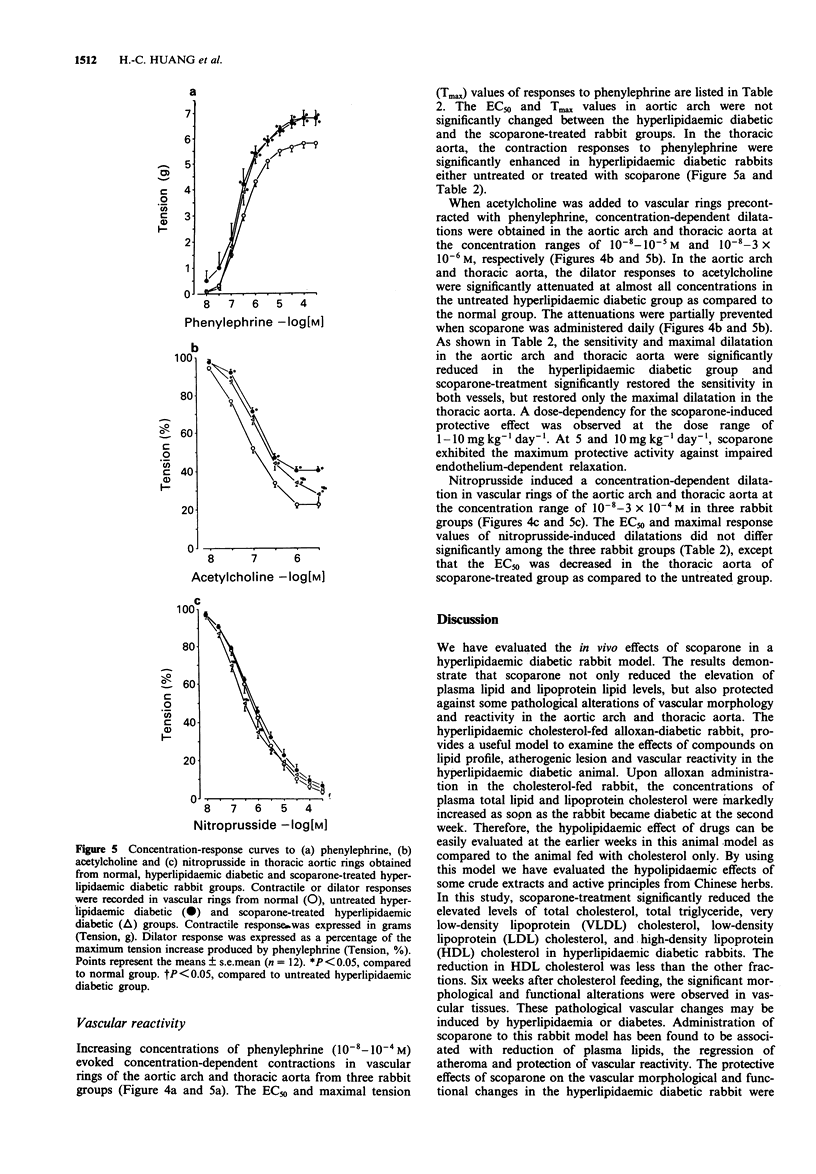
Abstract
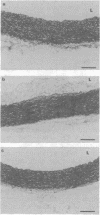
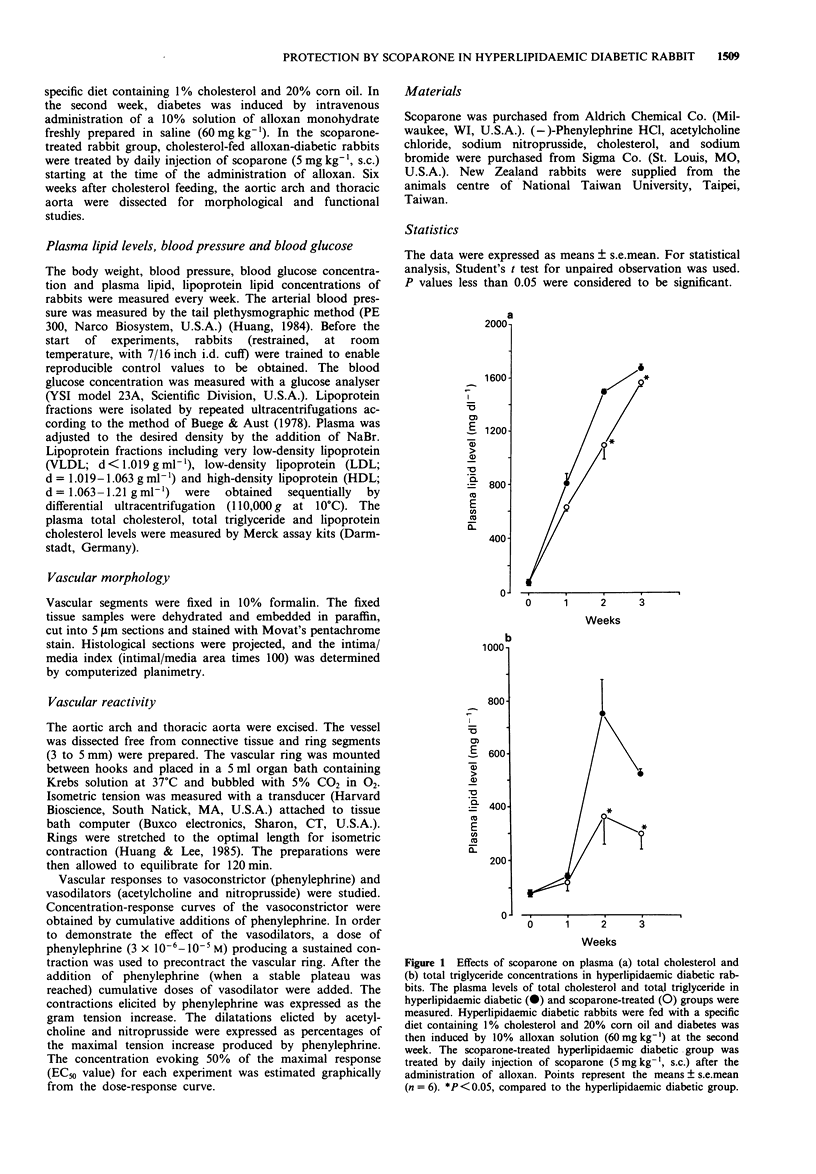
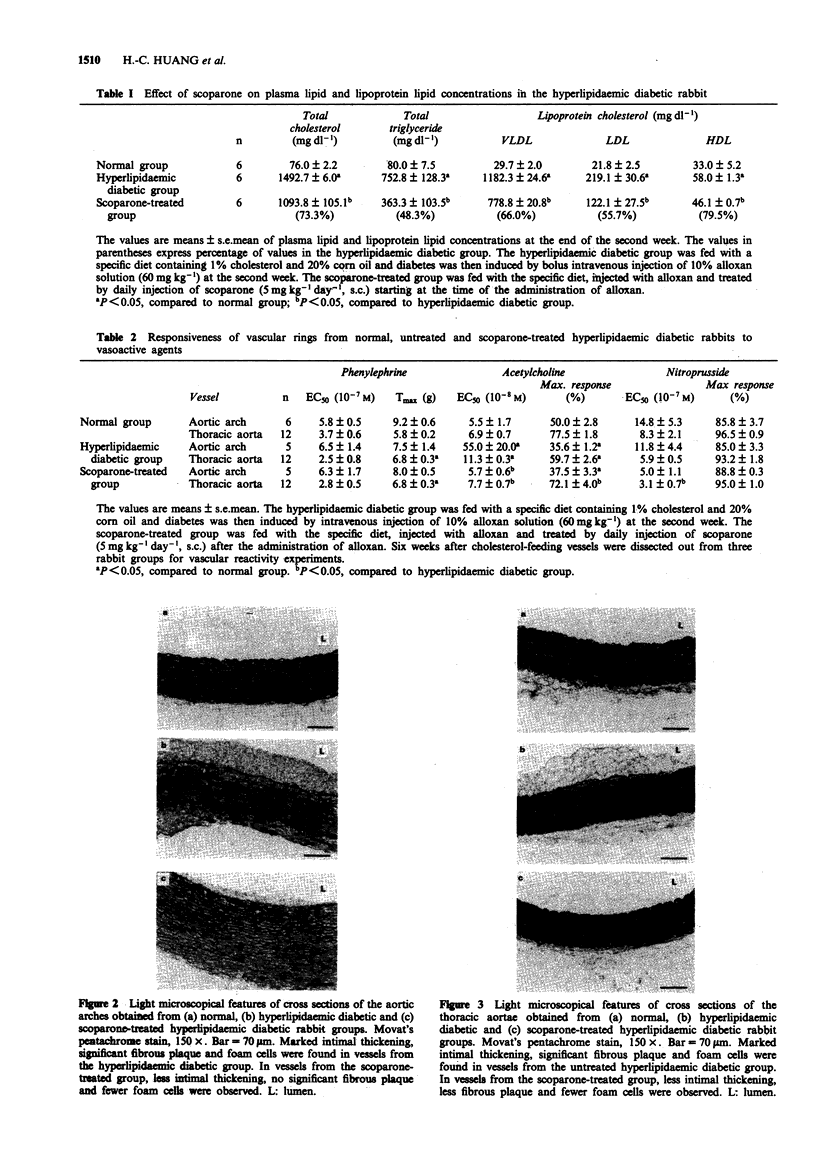
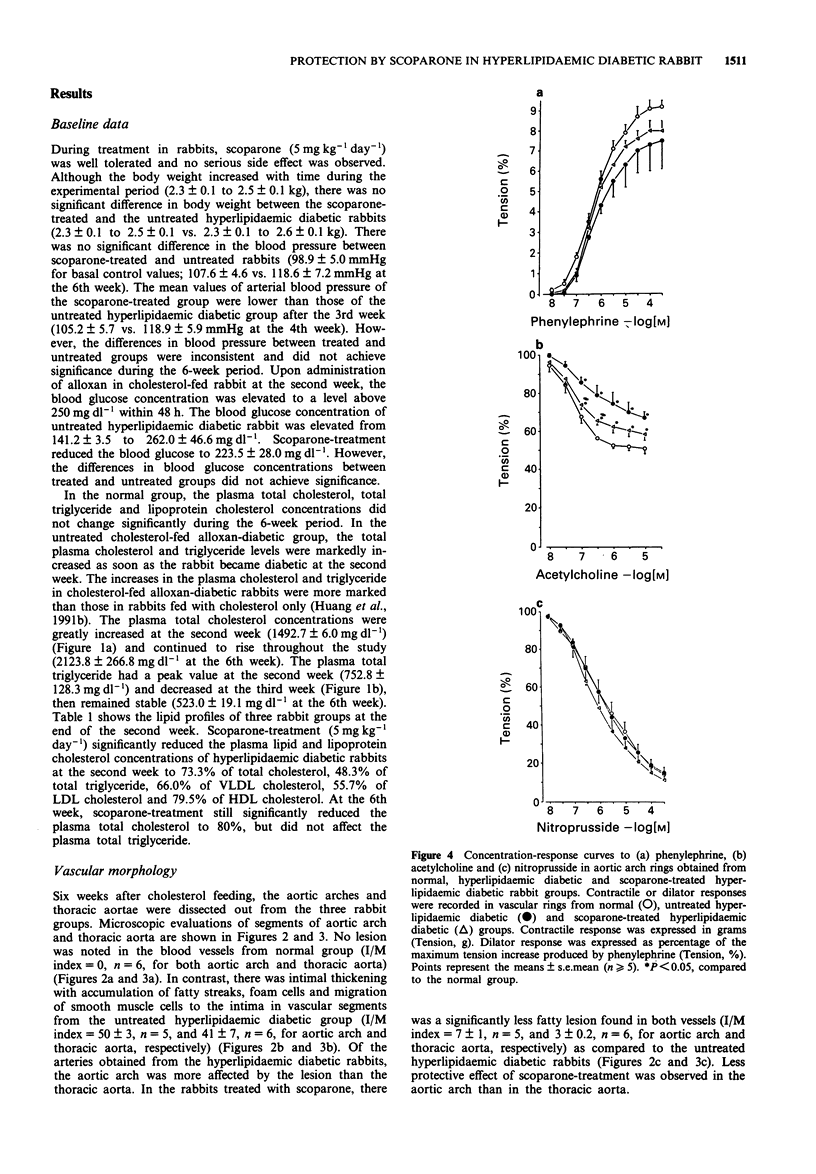
1. The in vivo pharmacological effects of scoparone (6,7-dimethoxycoumarin) in a hyperlipidaemic diabetic rabbit model were investigated. 2. Three groups of rabbits were studied: (1) normal, (2) hyperlipidaemic and diabetic-untreated and (3) hyperlipidaemic and diabetic-scoparone treated. The hyperlipidaemic diabetic rabbits were fed with 1% cholesterol and treated with alloxan, a diabetogenic agent. The plasma levels of total cholesterol, total triglyceride, very low-density lipoprotein (VLDL) cholesterol, low density lipoprotein (LDL) cholesterol and high-density lipoprotein (HDL) cholesterol were markedly increased as soon as the rabbit became diabetic at the second week. Scoparone-treatment (5 mg kg-1 day-1, s.c.) significantly reduced the plasma lipid and lipoprotein cholesterol levels of the hyperlipidaemic diabetic rabbit to 73.3% of total cholesterol, 48.3% of total triglyceride, 66.0% of VLDL cholesterol, 55.7% of LDL cholesterol and 79.5% of HDL cholesterol. 3. Six weeks after cholesterol-feeding, the aortic arch and thoracic aorta were dissected for morphological and functional studies. In vascular rings from the untreated hyperlipidaemic diabetic rabbit, there was intimal thickening with accumulation of fatty streaks, foam cells and migration of smooth muscle cells to the intima. In the rabbits treated with scoparone, there were fewer pathological morphology changes found in vascular segments than in the untreated hyperlipidaemic diabetic rabbits.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Angus J. A., Cocks T. M., Satoh K. The alpha adrenoceptors on endothelial cells. Fed Proc. 1986 Aug;45(9):2355–2359. [PubMed] [Google Scholar]
- Buege J. A., Aust S. D. Microsomal lipid peroxidation. Methods Enzymol. 1978;52:302–310. doi: 10.1016/s0076-6879(78)52032-6. [DOI] [PubMed] [Google Scholar]
- Cseuz R., Wenger T. L., Kunos G., Szentiványi M. Changes of adrenergic reaction pattern in experimental diabetes mellitus. Endocrinology. 1973 Sep;93(3):752–755. doi: 10.1210/endo-93-3-752. [DOI] [PubMed] [Google Scholar]
- Gebremedhin D., Koltai M. Z., Pogátsa G., Magyar K., Hadházy P. Differential contractile responsiveness of femoral arteries from healthy and diabetic dogs: role of endothelium. Arch Int Pharmacodyn Ther. 1987 Jul;288(1):100–108. [PubMed] [Google Scholar]
- Henry P. D., Yokoyama M. Supersensitivity of atherosclerotic rabbit aorta to ergonovine. Mediation by a serotonergic mechanism. J Clin Invest. 1980 Aug;66(2):306–313. doi: 10.1172/JCI109858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang H. C., Chu S. H., Chao P. D. Vasorelaxants from Chinese herbs, emodin and scoparone, possess immunosuppressive properties. Eur J Pharmacol. 1991 Jun 6;198(2-3):211–213. doi: 10.1016/0014-2999(91)90624-y. [DOI] [PubMed] [Google Scholar]
- Huang H. C. Effects of phospholipases A2 from Vipera russelli snake venom on blood pressure, plasma prostacyclin level and renin activity in rats. Toxicon. 1984;22(2):253–264. doi: 10.1016/0041-0101(84)90026-6. [DOI] [PubMed] [Google Scholar]
- Huang H. C., Huang Y. L., Chang J. H., Chen C. C., Lee Y. T. Possible mechanism of immunosuppressive effect of scoparone (6,7-dimethoxycoumarin). Eur J Pharmacol. 1992 Jul 7;217(2-3):143–148. doi: 10.1016/0014-2999(92)90835-r. [DOI] [PubMed] [Google Scholar]
- Huang H. C., Lai M. W., Wang H. R., Chung Y. L., Hsieh L. M., Chen C. C. Antiproliferative effect of esculetin on vascular smooth muscle cells: possible roles of signal transduction pathways. Eur J Pharmacol. 1993 Jun 11;237(1):39–44. doi: 10.1016/0014-2999(93)90090-5. [DOI] [PubMed] [Google Scholar]
- Huang H. C., Lee C. R., Weng Y. I., Lee M. C., Lee Y. T. Vasodilator effect of scoparone (6,7-dimethoxycoumarin) from a Chinese herb. Eur J Pharmacol. 1992 Jul 21;218(1):123–128. doi: 10.1016/0014-2999(92)90155-w. [DOI] [PubMed] [Google Scholar]
- Huang H. C., Lee C. Y. Relaxant effect of phospholipase A2 from Vipera russelli snake venom on rat aorta. Eur J Pharmacol. 1985 Nov 26;118(1-2):139–146. doi: 10.1016/0014-2999(85)90672-7. [DOI] [PubMed] [Google Scholar]
- Jayakody R. L., Senaratne M. P., Thomson A. B., Kappagoda C. T. Cholesterol feeding impairs endothelium-dependent relaxation of rabbit aorta. Can J Physiol Pharmacol. 1985 Sep;63(9):1206–1209. doi: 10.1139/y85-199. [DOI] [PubMed] [Google Scholar]
- Morel D. W., DiCorleto P. E., Chisolm G. M. Endothelial and smooth muscle cells alter low density lipoprotein in vitro by free radical oxidation. Arteriosclerosis. 1984 Jul-Aug;4(4):357–364. doi: 10.1161/01.atv.4.4.357. [DOI] [PubMed] [Google Scholar]
- Mügge A., Elwell J. H., Peterson T. E., Hofmeyer T. G., Heistad D. D., Harrison D. G. Chronic treatment with polyethylene-glycolated superoxide dismutase partially restores endothelium-dependent vascular relaxations in cholesterol-fed rabbits. Circ Res. 1991 Nov;69(5):1293–1300. doi: 10.1161/01.res.69.5.1293. [DOI] [PubMed] [Google Scholar]
- Parthasarathy S., Printz D. J., Boyd D., Joy L., Steinberg D. Macrophage oxidation of low density lipoprotein generates a modified form recognized by the scavenger receptor. Arteriosclerosis. 1986 Sep-Oct;6(5):505–510. doi: 10.1161/01.atv.6.5.505. [DOI] [PubMed] [Google Scholar]
- Sosenko J. M., Breslow J. L., Miettinen O. S., Gabbay K. H. Hyperglycemia and plasma lipid levels: a prospective study of young insulin-dependent diabetic patients. N Engl J Med. 1980 Mar 20;302(12):650–654. doi: 10.1056/NEJM198003203021202. [DOI] [PubMed] [Google Scholar]
- Tagawa H., Tomoike H., Nakamura M. Putative mechanisms of the impairment of endothelium-dependent relaxation of the aorta with atheromatous plaque in heritable hyperlipidemic rabbits. Circ Res. 1991 Feb;68(2):330–337. doi: 10.1161/01.res.68.2.330. [DOI] [PubMed] [Google Scholar]
- Yokoyama M., Akita H., Mizutani T., Fukuzaki H., Watanabe Y. Hyperreactivity of coronary arterial smooth muscles in response to ergonovine from rabbits with hereditary hyperlipidemia. Circ Res. 1983 Jul;53(1):63–71. doi: 10.1161/01.res.53.1.63. [DOI] [PubMed] [Google Scholar]