Abstract
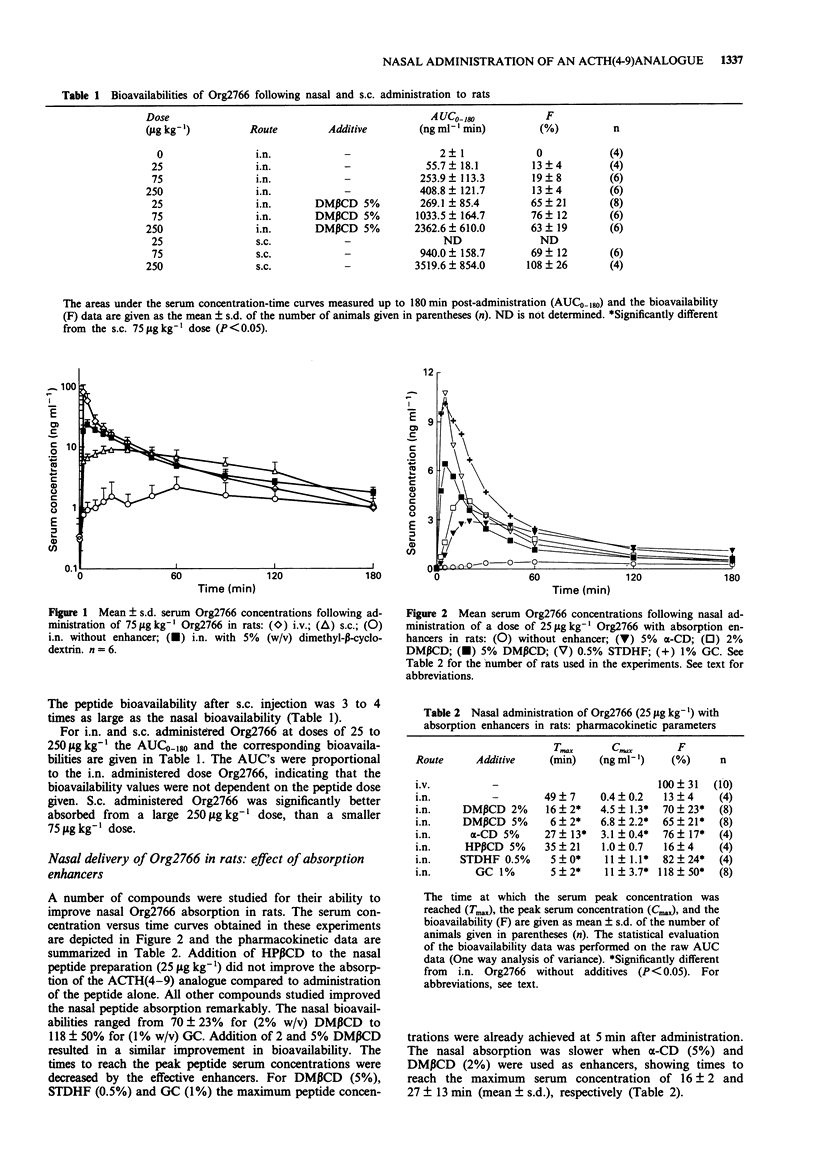
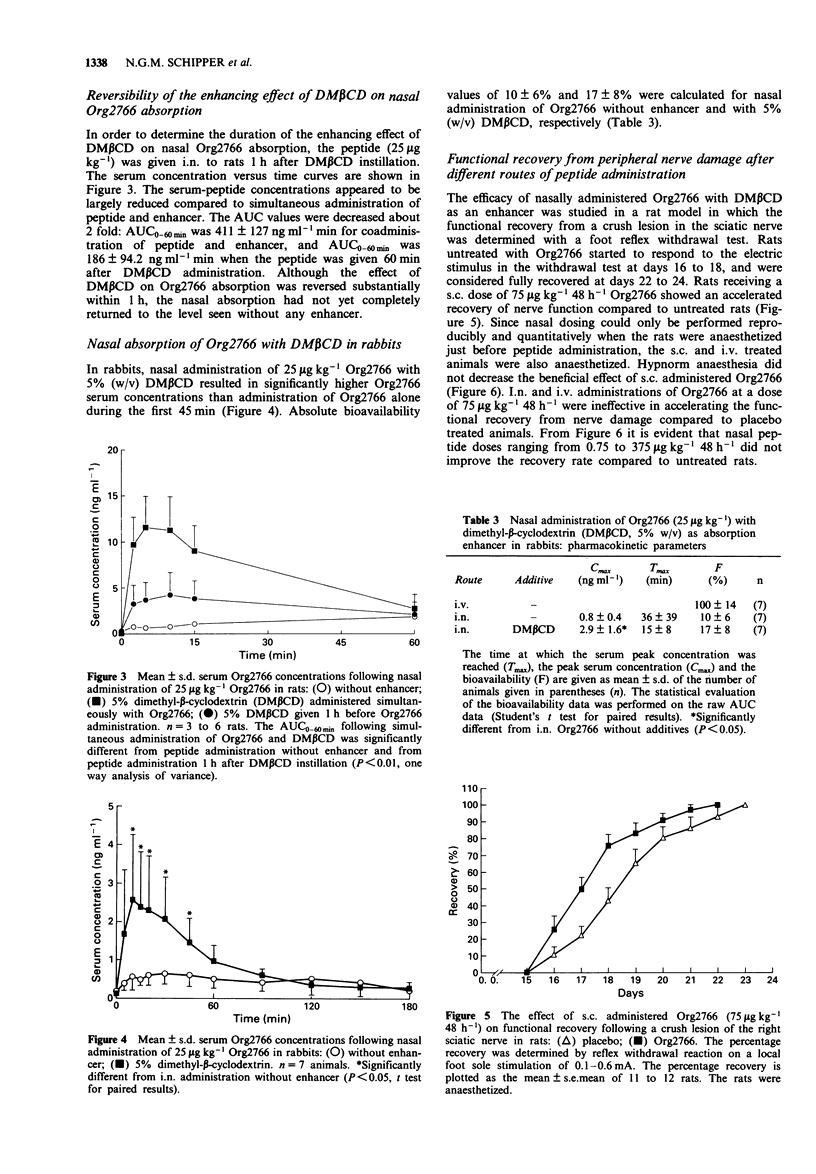
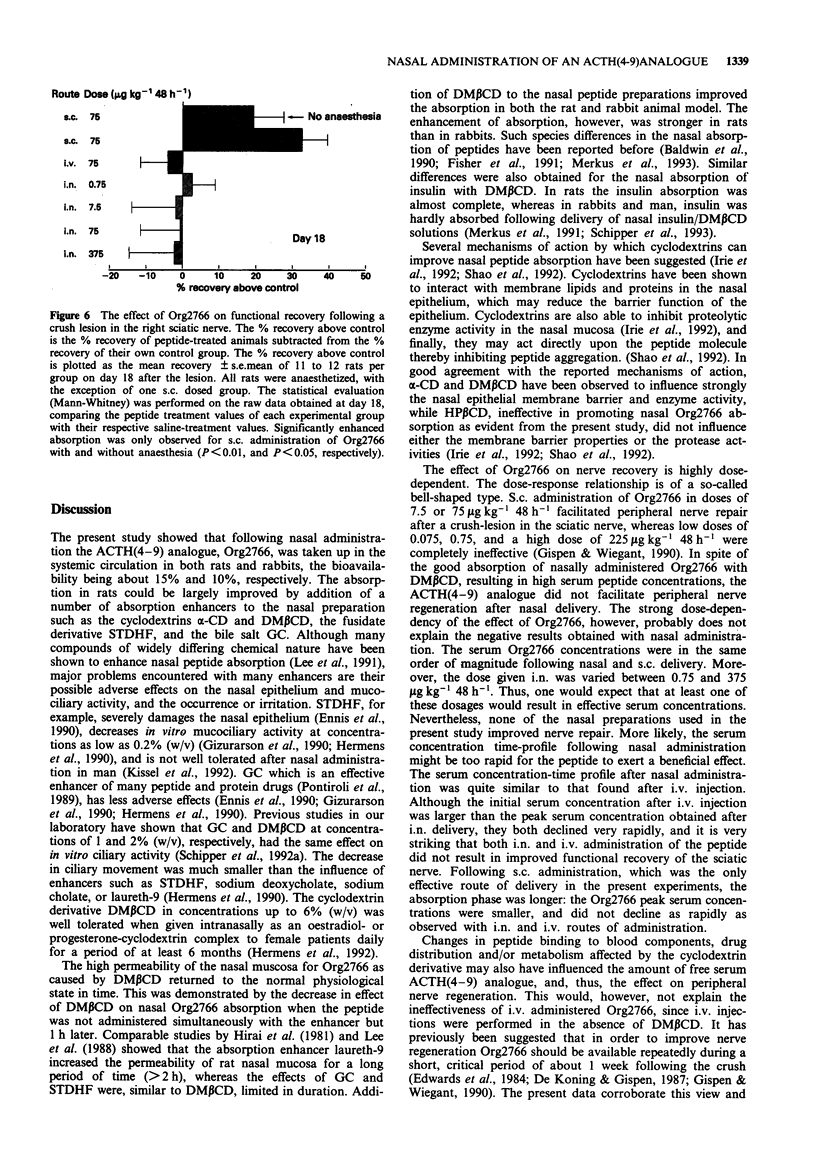
1. The systemic absorption and the neurotrophic effect of the metabolically stabilized ACTH (4-9) analogue, Org2766, were investigated following intranasal (i.n.) administration. 2. Without additives the nasal bioavailability of the peptide was in the order of 15 and 10% in rats and rabbits, respectively. The absorption could be improved by addition of a variety of absorption enhancers to the nasal preparation. The beta-cyclodextrin derivative, dimethyl-beta-cyclodextrin (DM beta CD) at a concentration of 5% (w/v) improved the absorption in rats about 5 fold from 13 +/- 4% (mean +/- s.d.) for administration of the peptide alone to 65 +/- 21%, and in rabbits 1 to 2 fold, from 10 +/- 6% to 17 +/- 8%. 3. The increased permeability of the rat nasal mucosa for Org2766 caused by DM beta CD in rats reversed substantially within 1 h. However, the nasal absorption had not yet completely returned to the level without enhancer. 4. S.c. administered Org2766 accelerated the functional recovery from peripheral nerve damage in rats. However, the peptide did not facilitate nerve repair following i.n. administration with DM beta CD, in spite of the fact that Org2766 was well absorbed. I.v. injection of Org2766 was also ineffective.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baldwin P. A., Klingbeil C. K., Grimm C. J., Longenecker J. P. The effect of sodium tauro-24,25-dihydrofusidate on the nasal absorption of human growth hormone in three animal models. Pharm Res. 1990 May;7(5):547–552. doi: 10.1023/a:1015885204249. [DOI] [PubMed] [Google Scholar]
- De Koning P., Brakkee J. H., Gispen W. H. Methods for producing a reproducible crush in the sciatic and tibial nerve of the rat and rapid and precise testing of return of sensory function. Beneficial effects of melanocortins. J Neurol Sci. 1986 Jul;74(2-3):237–246. doi: 10.1016/0022-510x(86)90109-7. [DOI] [PubMed] [Google Scholar]
- De Koning P., Gispen W. H. Org.2766 improves functional and electrophysiological aspects of regenerating sciatic nerve in the rat. Peptides. 1987 May-Jun;8(3):415–422. doi: 10.1016/0196-9781(87)90003-9. [DOI] [PubMed] [Google Scholar]
- Dekker A. J., Princen M. M., De Nijs H., De Leede L. G., Broekkamp C. L. Acceleration of recovery from sciatic nerve damage by the ACTH (4-9) analog Org 2766: different routes of administration. Peptides. 1987 Nov-Dec;8(6):1057–1059. doi: 10.1016/0196-9781(87)90136-7. [DOI] [PubMed] [Google Scholar]
- Edwards P. M., Van der Zee C. E., Verhaagen J., Schotman P., Jennekens F. G., Gispen W. H. Evidence that the neurotrophic actions of alpha-MSH may derive from its ability to mimick the actions of a peptide formed in degenerating nerve stumps. J Neurol Sci. 1984 Jun;64(3):333–340. doi: 10.1016/0022-510x(84)90181-3. [DOI] [PubMed] [Google Scholar]
- Ennis R. D., Borden L., Lee W. A. The effects of permeation enhancers on the surface morphology of the rat nasal mucosa: a scanning electron microscopy study. Pharm Res. 1990 May;7(5):468–475. doi: 10.1023/a:1015856430657. [DOI] [PubMed] [Google Scholar]
- Hermens W. A., Belder C. W., Merkus J. M., Hooymans P. M., Verhoef J., Merkus F. W. Intranasal administration of estradiol in combination with progesterone to oophorectomized women: a pilot study. Eur J Obstet Gynecol Reprod Biol. 1992 Jan 9;43(1):65–70. doi: 10.1016/0028-2243(92)90245-t. [DOI] [PubMed] [Google Scholar]
- Hermens W. A., Hooymans P. M., Verhoef J. C., Merkus F. W. Effects of absorption enhancers on human nasal tissue ciliary movement in vitro. Pharm Res. 1990 Feb;7(2):144–146. doi: 10.1023/a:1015872617511. [DOI] [PubMed] [Google Scholar]
- Kissel T., Drewe J., Bantle S., Rummelt A., Beglinger C. Tolerability and absorption enhancement of intranasally administered octreotide by sodium taurodihydrofusidate in healthy subjects. Pharm Res. 1992 Jan;9(1):52–57. doi: 10.1023/a:1018927710280. [DOI] [PubMed] [Google Scholar]
- Lee V. H., Yamamoto A., Kompella U. B. Mucosal penetration enhancers for facilitation of peptide and protein drug absorption. Crit Rev Ther Drug Carrier Syst. 1991;8(2):91–192. [PubMed] [Google Scholar]
- Merkus F. W., Verhoef J. C., Romeijn S. G., Schipper N. G. Absorption enhancing effect of cyclodextrins on intranasally administered insulin in rats. Pharm Res. 1991 May;8(5):588–592. doi: 10.1023/a:1015896405389. [DOI] [PubMed] [Google Scholar]
- Pontiroli A. E., Calderara A., Pozza G. Intranasal drug delivery. Potential advantages and limitations from a clinical pharmacokinetic perspective. Clin Pharmacokinet. 1989 Nov;17(5):299–307. doi: 10.2165/00003088-198917050-00001. [DOI] [PubMed] [Google Scholar]
- Schipper N. G., Romeijn S. G., Verhoef J. C., Merkus F. W. Nasal insulin delivery with dimethyl-beta-cyclodextrin as an absorption enhancer in rabbits: powder more effective than liquid formulations. Pharm Res. 1993 May;10(5):682–686. doi: 10.1023/a:1018999414088. [DOI] [PubMed] [Google Scholar]
- Shao Z., Krishnamoorthy R., Mitra A. K. Cyclodextrins as nasal absorption promoters of insulin: mechanistic evaluations. Pharm Res. 1992 Sep;9(9):1157–1163. doi: 10.1023/a:1015847604654. [DOI] [PubMed] [Google Scholar]
- Spruijt B., Pitsikas N., Algeri S., Gispen W. H. Org2766 improves performance of rats with unilateral lesions in the fimbria fornix in a spatial learning task. Brain Res. 1990 Sep 17;527(2):192–197. doi: 10.1016/0006-8993(90)91137-6. [DOI] [PubMed] [Google Scholar]
- Strand F. L., Rose K. J., Zuccarelli L. A., Kume J., Alves S. E., Antonawich F. J., Garrett L. Y. Neuropeptide hormones as neurotrophic factors. Physiol Rev. 1991 Oct;71(4):1017–1046. doi: 10.1152/physrev.1991.71.4.1017. [DOI] [PubMed] [Google Scholar]
- Van der Zee C. E., Brakkee J. H., Gispen W. H. Putative neurotrophic factors and functional recovery from peripheral nerve damage in the rat. Br J Pharmacol. 1991 May;103(1):1041–1046. doi: 10.1111/j.1476-5381.1991.tb12297.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Zee C. E., Brakkee J. H., Gispen W. H. alpha-MSH and Org.2766 in peripheral nerve regeneration: different routes of delivery. Eur J Pharmacol. 1988 Mar 15;147(3):351–357. doi: 10.1016/0014-2999(88)90168-9. [DOI] [PubMed] [Google Scholar]
- Wolterink G., Van Zanten E., Kamsteeg H., Radhakishun F. S., Van Ree J. M. Functional recovery after destruction of dopamine systems in the nucleus accumbens of rats. II. Facilitation by the ACTH-(4-9) analog ORG 2766. Brain Res. 1990 Jan 15;507(1):101–108. doi: 10.1016/0006-8993(90)90527-i. [DOI] [PubMed] [Google Scholar]
- van der Hoop R. G., Vecht C. J., van der Burg M. E., Elderson A., Boogerd W., Heimans J. J., Vries E. P., van Houwelingen J. C., Jennekens F. G., Gispen W. H. Prevention of cisplatin neurotoxicity with an ACTH(4-9) analogue in patients with ovarian cancer. N Engl J Med. 1990 Jan 11;322(2):89–94. doi: 10.1056/NEJM199001113220204. [DOI] [PubMed] [Google Scholar]


