Abstract
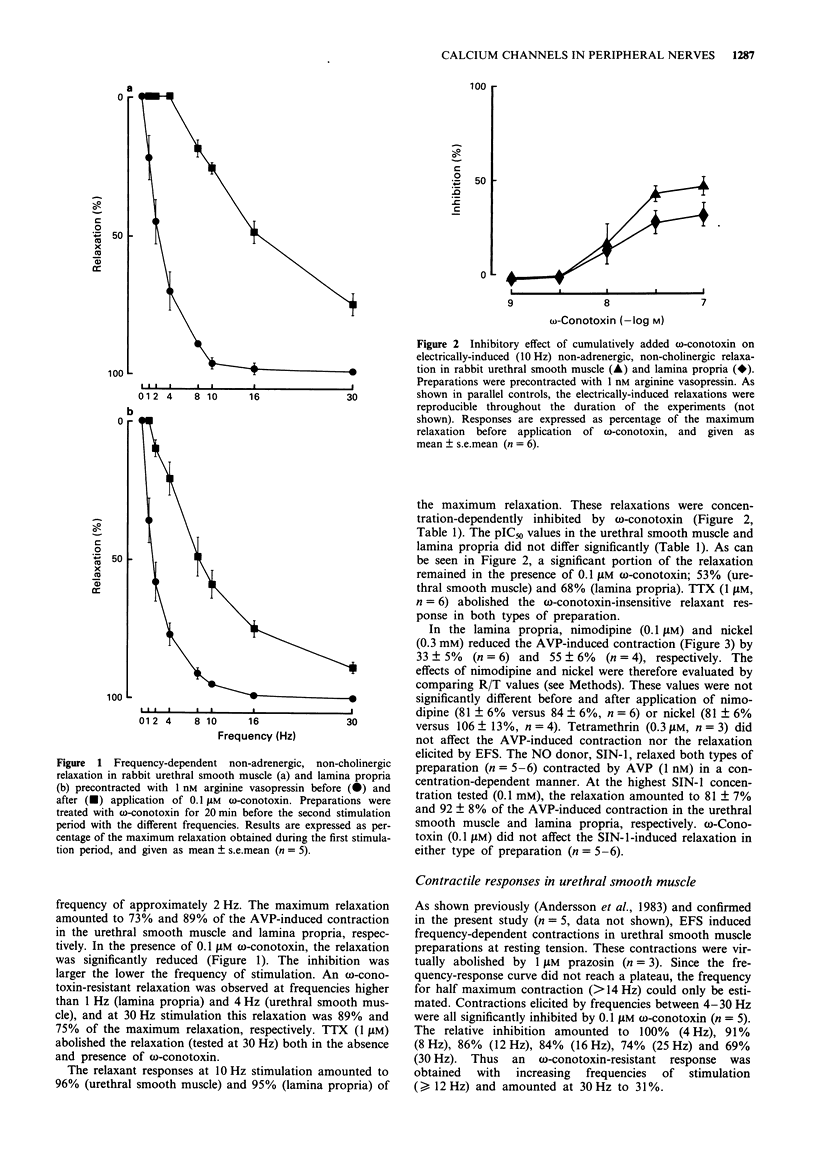
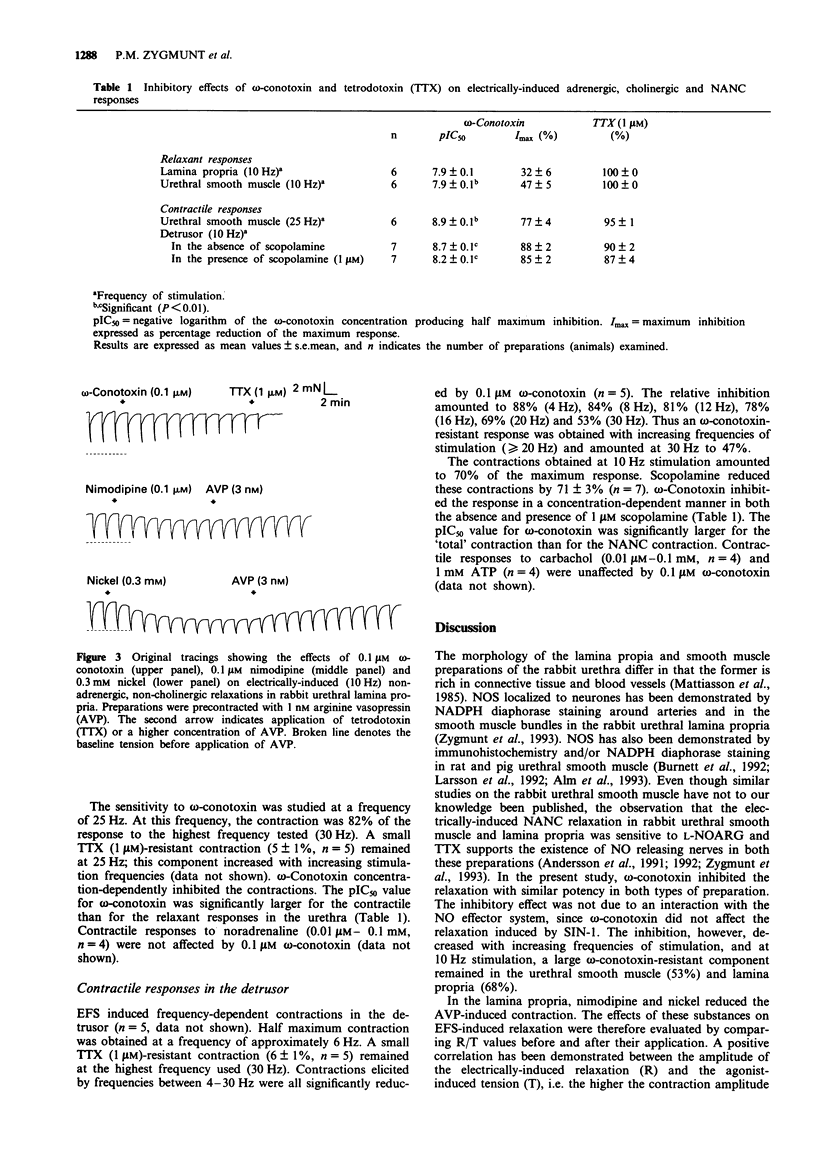
1. The effects of omega-conotoxin GVIA (an inhibitor of N-type voltage-operated calcium channels; VOCCs) were compared on adrenergic, cholinergic and non-adrenergic, non-cholinergic (NANC) responses induced by electrical field stimulation (EFS) in the rabbit urethra and detrusor. 2. EFS induced a relaxation in urethral smooth muscle and lamina propria precontracted by arginine vasopressin (AVP). The relaxation was abolished by tetrodotoxin (TTX) or the nitric oxide (NO) synthase inhibitor N omega-nitro-L-arginine. omega-Conotoxin inhibited the relaxation induced by EFS, but not that elicited by the NO donor 3-morpholino-sydnonimin. The inhibition, however, decreased with increasing frequencies of stimulation. Nimodipine, tetramethrin and nickel did not affect the omega-contoxin-resistant relaxation in lamina propria, suggesting that neuronal L or T VOCCs were not involved in the response. 3. EFS contracted urethral smooth muscle at resting tension. The contractions were virtually abolished by TTX or prazosin. omega-Conotoxin effectively inhibited the contractile responses to EFS, but not those to exogenous noradrenaline. An omega-conotoxin-resistant contraction was, however, observed at high frequencies of stimulation. 4. The detrusor responded with frequency-dependent contractions upon EFS. A TTX-resistant contraction less than 10% of controls remained at 30 Hz stimulation. At a stimulation frequency of 10 Hz, scopolamine reduced the EFS-induced contraction by 71%. omega-Conotoxin inhibited the responses in both the absence and presence of scopolamine. The inhibition decreased with increasing frequencies of stimulation (examined in the absence of scopolamine). omega-Conotoxin did not affect the contractile responses to carbachol or adenosine 5'-triphosphate.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alm P., Larsson B., Ekblad E., Sundler F., Andersson K. E. Immunohistochemical localization of peripheral nitric oxide synthase-containing nerves using antibodies raised against synthesized C- and N-terminal fragments of a cloned enzyme from rat brain. Acta Physiol Scand. 1993 Aug;148(4):421–429. doi: 10.1111/j.1748-1716.1993.tb09578.x. [DOI] [PubMed] [Google Scholar]
- Ambache N., Zar M. A. Non-cholinergic transmission by post-ganglionic motor neurones in the mammalian bladder. J Physiol. 1970 Oct;210(3):761–783. doi: 10.1113/jphysiol.1970.sp009240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson K. E., Garcia Pascual A., Forman A., Tøttrup A. Non-adrenergic, non-cholinergic nerve-mediated relaxation of rabbit urethra is caused by nitric oxide. Acta Physiol Scand. 1991 Jan;141(1):133–134. doi: 10.1111/j.1748-1716.1991.tb09056.x. [DOI] [PubMed] [Google Scholar]
- Andersson K. E., Garcia Pascual A., Persson K., Forman A., Tøttrup A. Electrically-induced, nerve-mediated relaxation of rabbit urethra involves nitric oxide. J Urol. 1992 Jan;147(1):253–259. doi: 10.1016/s0022-5347(17)37208-7. [DOI] [PubMed] [Google Scholar]
- Belai A., Ralevic V., Burnstock G. VIP release from enteric nerves is independent of extracellular calcium. Regul Pept. 1987 Oct;19(1-2):79–89. doi: 10.1016/0167-0115(87)90077-2. [DOI] [PubMed] [Google Scholar]
- Bredt D. S., Hwang P. M., Glatt C. E., Lowenstein C., Reed R. R., Snyder S. H. Cloned and expressed nitric oxide synthase structurally resembles cytochrome P-450 reductase. Nature. 1991 Jun 27;351(6329):714–718. doi: 10.1038/351714a0. [DOI] [PubMed] [Google Scholar]
- Burnett A. L., Lowenstein C. J., Bredt D. S., Chang T. S., Snyder S. H. Nitric oxide: a physiologic mediator of penile erection. Science. 1992 Jul 17;257(5068):401–403. doi: 10.1126/science.1378650. [DOI] [PubMed] [Google Scholar]
- Fox A. P., Nowycky M. C., Tsien R. W. Kinetic and pharmacological properties distinguishing three types of calcium currents in chick sensory neurones. J Physiol. 1987 Dec;394:149–172. doi: 10.1113/jphysiol.1987.sp016864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Förstermann U., Schmidt H. H., Pollock J. S., Sheng H., Mitchell J. A., Warner T. D., Nakane M., Murad F. Isoforms of nitric oxide synthase. Characterization and purification from different cell types. Biochem Pharmacol. 1991 Oct 24;42(10):1849–1857. doi: 10.1016/0006-2952(91)90581-o. [DOI] [PubMed] [Google Scholar]
- Garcia-Pascual A., Costa G., Garcia-Sacristan A., Andersson K. E. Relaxation of sheep urethral muscle induced by electrical stimulation of nerves: involvement of nitric oxide. Acta Physiol Scand. 1991 Apr;141(4):531–539. doi: 10.1111/j.1748-1716.1991.tb09114.x. [DOI] [PubMed] [Google Scholar]
- Hagiwara N., Irisawa H., Kameyama M. Contribution of two types of calcium currents to the pacemaker potentials of rabbit sino-atrial node cells. J Physiol. 1988 Jan;395:233–253. doi: 10.1113/jphysiol.1988.sp016916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hata F., Fujita A., Saeki K., Kishi I., Takeuchi T., Yagasaki O. Selective inhibitory effects of calcium channel antagonists on the two components of the neurogenic response of guinea pig vas deferens. J Pharmacol Exp Ther. 1992 Oct;263(1):214–220. [PubMed] [Google Scholar]
- Igawa Y., Mattiasson A., Andersson K. E. Functional importance of cholinergic and purinergic neurotransmission for micturition contraction in the normal, unanaesthetized rat. Br J Pharmacol. 1993 Jun;109(2):473–479. doi: 10.1111/j.1476-5381.1993.tb13593.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ignarro L. J. Endothelium-derived nitric oxide: actions and properties. FASEB J. 1989 Jan;3(1):31–36. doi: 10.1096/fasebj.3.1.2642868. [DOI] [PubMed] [Google Scholar]
- Keith R. A., Mangano T. J., Pacheco M. A., Salama A. I. Characterization of the effects of omega-conotoxin GVIA on the responses of voltage-sensitive calcium channels. J Auton Pharmacol. 1989 Aug;9(4):243–252. doi: 10.1111/j.1474-8673.1989.tb00215.x. [DOI] [PubMed] [Google Scholar]
- Larsson B., Högestätt E. D., Mattiasson A., Andersson K. E. Differential effects of nifedipine, verapamil, and diltiazem on noradrenaline-induced contractions, adrenergic transmitter release, and alpha-adrenoceptor binding in the female rabbit urethra. Naunyn Schmiedebergs Arch Pharmacol. 1984 May;326(1):14–21. doi: 10.1007/BF00518773. [DOI] [PubMed] [Google Scholar]
- Llinás R., Sugimori M., Lin J. W., Cherksey B. Blocking and isolation of a calcium channel from neurons in mammals and cephalopods utilizing a toxin fraction (FTX) from funnel-web spider poison. Proc Natl Acad Sci U S A. 1989 Mar;86(5):1689–1693. doi: 10.1073/pnas.86.5.1689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundy P. M., Hong A., Frew R. Inhibition of a dihydropyridine, omega-conotoxin insensitive Ca2+ channel in rat synaptosomes by venom of the spider Hololena curta. Eur J Pharmacol. 1992 Jan 14;225(1):51–56. doi: 10.1016/0922-4106(92)90038-w. [DOI] [PubMed] [Google Scholar]
- Maggi C. A. Omega conotoxin and prejunctional modulation of the biphasic response of the rat isolated urinary bladder to single pulse electrical field stimulation. J Auton Pharmacol. 1991 Oct;11(5):295–304. doi: 10.1111/j.1474-8673.1991.tb00253.x. [DOI] [PubMed] [Google Scholar]
- Mattiasson A., Andersson K. E., Sjögren C. Contractant and relaxant properties of the female rabbit urethral submucosa. J Urol. 1985 Feb;133(2):304–310. doi: 10.1016/s0022-5347(17)48928-2. [DOI] [PubMed] [Google Scholar]
- Minchin M. C. The role of Ca2+ in the protoveratrine-induced release of gamma-aminobutyrate from rat brain slices. Biochem J. 1980 Aug 15;190(2):333–339. doi: 10.1042/bj1900333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murad F., Forstermann U., Nakane M., Schmidt H., Pollock J., Sheng H., Matsumoto T., Warner T., Mitchell J., Tracey R. The nitric oxide-cyclic GMP signal transduction pathway in vascular smooth muscle preparations and other tissues. Jpn J Pharmacol. 1992;58 (Suppl 2):150P–157P. [PubMed] [Google Scholar]
- Myers P. R., Minor R. L., Jr, Guerra R., Jr, Bates J. N., Harrison D. G. Vasorelaxant properties of the endothelium-derived relaxing factor more closely resemble S-nitrosocysteine than nitric oxide. Nature. 1990 May 10;345(6271):161–163. doi: 10.1038/345161a0. [DOI] [PubMed] [Google Scholar]
- Mülsch A., Busse R. NG-nitro-L-arginine (N5-[imino(nitroamino)methyl]-L-ornithine) impairs endothelium-dependent dilations by inhibiting cytosolic nitric oxide synthesis from L-arginine. Naunyn Schmiedebergs Arch Pharmacol. 1990 Jan-Feb;341(1-2):143–147. doi: 10.1007/BF00195071. [DOI] [PubMed] [Google Scholar]
- Persson K., Andersson K. E. Nitric oxide and relaxation of pig lower urinary tract. Br J Pharmacol. 1992 Jun;106(2):416–422. doi: 10.1111/j.1476-5381.1992.tb14349.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruneau D., Angus J. A. Omega-conotoxin GVIA is a potent inhibitor of sympathetic neurogenic responses in rat small mesenteric arteries. Br J Pharmacol. 1990 May;100(1):180–184. doi: 10.1111/j.1476-5381.1990.tb12073.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandoval M. E. Sodium-dependent efflux of [3H]GABA from synaptosomes probably related to mitochondrial calcium mobilization. J Neurochem. 1980 Oct;35(4):915–921. doi: 10.1111/j.1471-4159.1980.tb07090.x. [DOI] [PubMed] [Google Scholar]
- Schoffelmeer A. N., Mulder A. H. [3H]noradrenaline release from brain slices induced by an increase in the intracellular sodium concentration: role of intracellular calcium stores. J Neurochem. 1983 Mar;40(3):615–621. doi: 10.1111/j.1471-4159.1983.tb08025.x. [DOI] [PubMed] [Google Scholar]
- Sher E., Biancardi E., Passafaro M., Clementi F. Physiopathology of neuronal voltage-operated calcium channels. FASEB J. 1991 Sep;5(12):2677–2683. doi: 10.1096/fasebj.5.12.1655547. [DOI] [PubMed] [Google Scholar]
- Sneddon P., Graham A. Role of nitric oxide in the autonomic innervation of smooth muscle. J Auton Pharmacol. 1992 Dec;12(6):445–456. doi: 10.1111/j.1474-8673.1992.tb00393.x. [DOI] [PubMed] [Google Scholar]
- Snutch T. P., Reiner P. B. Ca2+ channels: diversity of form and function. Curr Opin Neurobiol. 1992 Jun;2(3):247–253. doi: 10.1016/0959-4388(92)90111-w. [DOI] [PubMed] [Google Scholar]
- Snyder S. H. Nitric oxide: first in a new class of neurotransmitters. Science. 1992 Jul 24;257(5069):494–496. doi: 10.1126/science.1353273. [DOI] [PubMed] [Google Scholar]
- Uchitel O. D., Protti D. A., Sanchez V., Cherksey B. D., Sugimori M., Llinás R. P-type voltage-dependent calcium channel mediates presynaptic calcium influx and transmitter release in mammalian synapses. Proc Natl Acad Sci U S A. 1992 Apr 15;89(8):3330–3333. doi: 10.1073/pnas.89.8.3330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanin A. F. Endothelium-derived relaxing factor is a nitrosyl iron complex with thiol ligands. FEBS Lett. 1991 Sep 2;289(1):1–3. doi: 10.1016/0014-5793(91)80894-9. [DOI] [PubMed] [Google Scholar]
- Vedernikov Y. P., Mordvintcev P. I., Malenkova I. V., Vanin A. F. Similarity between the vasorelaxing activity of dinitrosyl iron cysteine complexes and endothelium-derived relaxing factor. Eur J Pharmacol. 1992 Feb 18;211(3):313–317. doi: 10.1016/0014-2999(92)90386-i. [DOI] [PubMed] [Google Scholar]
- Zygmunt P. K., Persson K., Alm P., Larsson B., Andersson K. E. The L-arginine/nitric oxide pathway in the rabbit urethral lamina propria. Acta Physiol Scand. 1993 Aug;148(4):431–439. doi: 10.1111/j.1748-1716.1993.tb09579.x. [DOI] [PubMed] [Google Scholar]
- Zygmunt P. M., Högestätt E. D. Calcium channels at the adrenergic neuroeffector junction in the rabbit ear artery. Naunyn Schmiedebergs Arch Pharmacol. 1993 Jun;347(6):617–623. doi: 10.1007/BF00166944. [DOI] [PubMed] [Google Scholar]


