Abstract
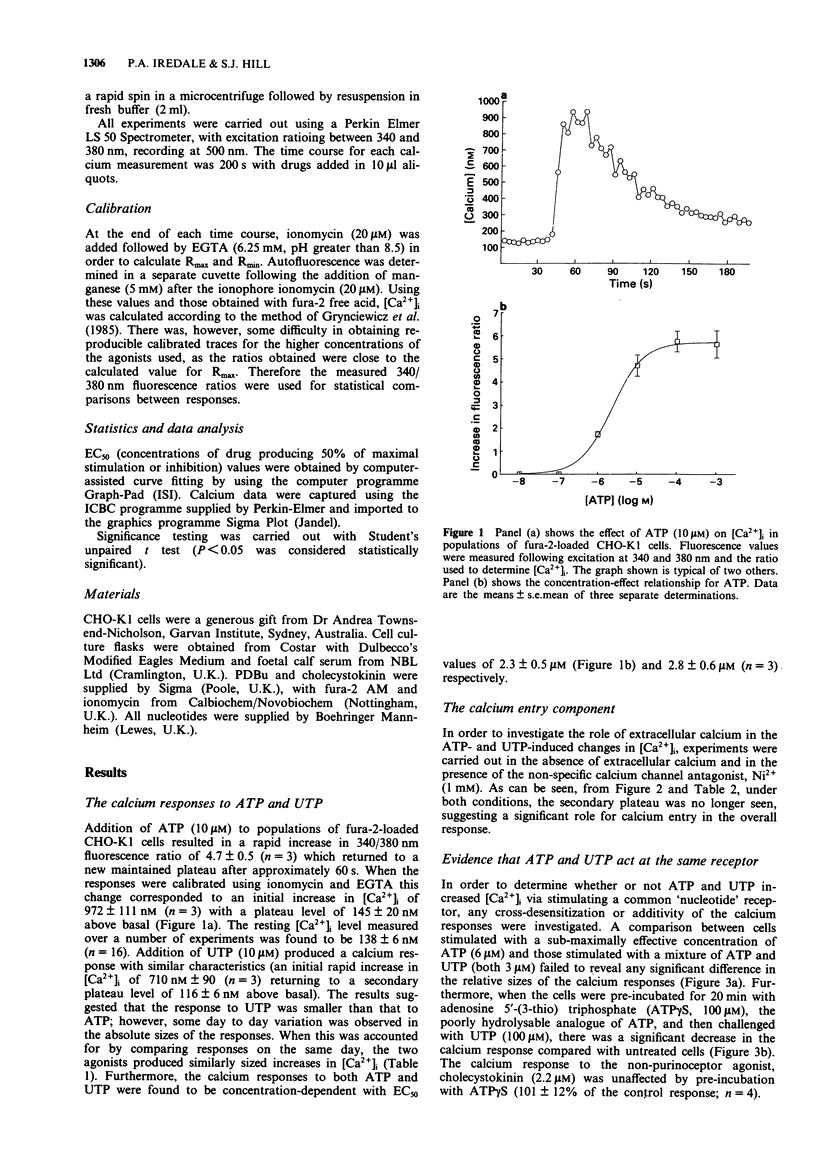
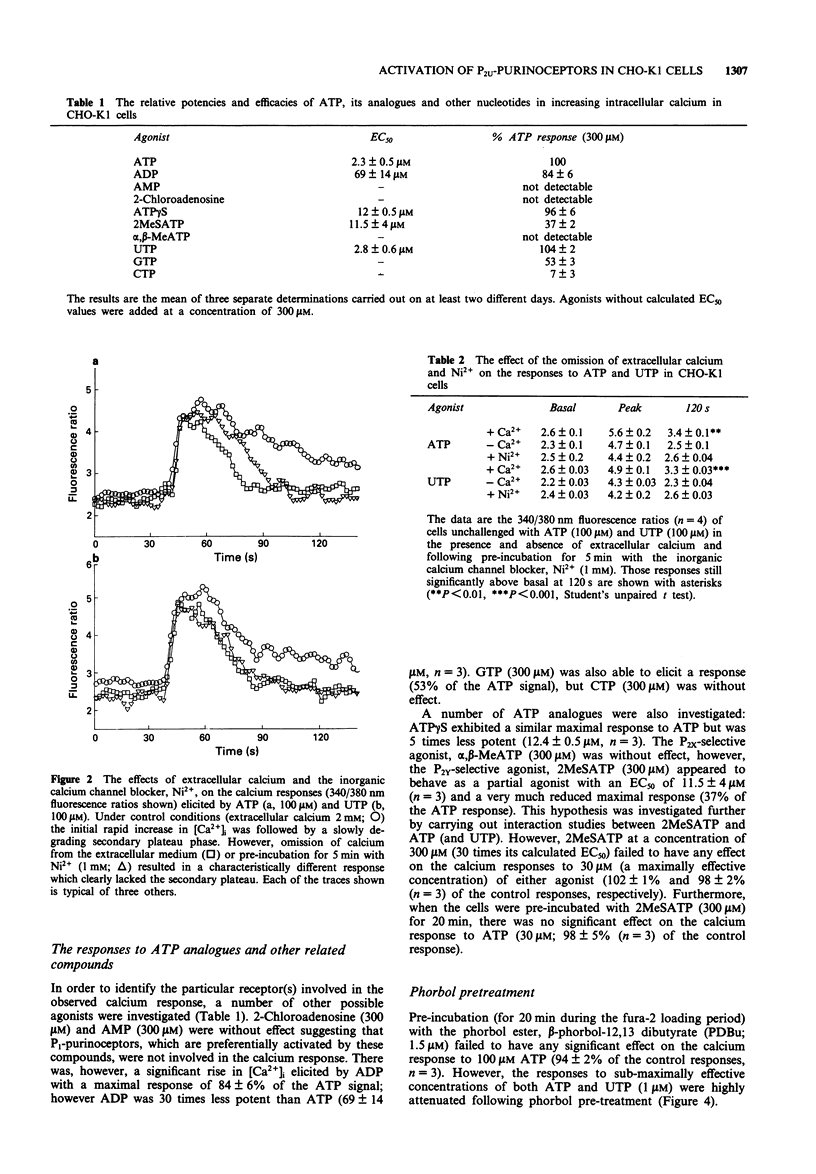
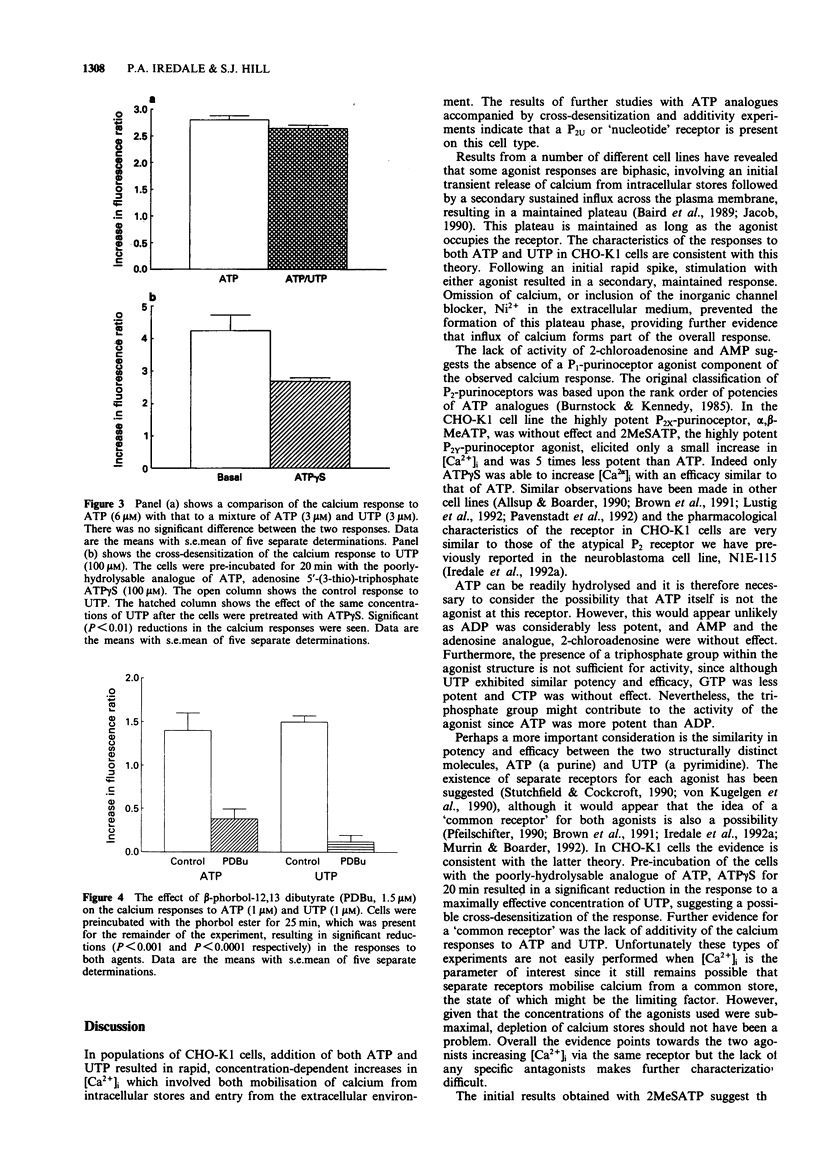
1. Increases in intracellular calcium ([Ca2+]i) were measured in chinese hamster cultured ovary cells (clone, CHO-K1), by use of the fluorescent, calcium-sensitive dye, fura-2. 2. Addition of both ATP and UTP elicited rapid increases in [Ca2+]i due to mobilization from intracellular stores and calcium entry across the plasma membrane. 3. Omission of calcium from the extracellular medium and pre-incubation with the inorganic calcium channel blocker, nickel (Ni2+) prevented the calcium entry components of the responses. 4. Investigation of the concentration-response relationships of various analogues of ATP suggests the presence of a purinoceptor which cannot be characterized as P2X or P2Y. In addition, there appears to be a sub-population of P2Y-purinoceptors which do not cross-react with the 'nucleotide' receptor population. 5. Cross-desensitization and additivity experiments suggest that both ATP and UTP activate the same receptor. 6. Pre-incubation with the tumour-promoting agent, beta-phorbol-12,13 dibutyrate (PDBu), caused a reduction in the increases in [Ca2+]i, suggesting a role for protein kinase C in feedback inhibition of purinoceptor responses in this cell line. 7. In summary, we present evidence for the existence of an endogenous P2U-purinoceptor (or 'nucleotide receptor') which is linked to increases in [Ca2+]i in CHO-K1 cells.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allsup D. J., Boarder M. R. Comparison of P2 purinergic receptors of aortic endothelial cells with those of adrenal medulla: evidence for heterogeneity of receptor subtype and of inositol phosphate response. Mol Pharmacol. 1990 Jul;38(1):84–91. [PubMed] [Google Scholar]
- Baird J. G., Lambert D. G., McBain J., Nahorski S. R. Muscarinic receptors coupled to phosphoinositide hydrolysis and elevated cytosolic calcium in a human neuroblastoma cell line SK-N-SH. Br J Pharmacol. 1989 Dec;98(4):1328–1334. doi: 10.1111/j.1476-5381.1989.tb12681.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown H. A., Lazarowski E. R., Boucher R. C., Harden T. K. Evidence that UTP and ATP regulate phospholipase C through a common extracellular 5'-nucleotide receptor in human airway epithelial cells. Mol Pharmacol. 1991 Nov;40(5):648–655. [PubMed] [Google Scholar]
- Burnstock G., Kennedy C. Is there a basis for distinguishing two types of P2-purinoceptor? Gen Pharmacol. 1985;16(5):433–440. doi: 10.1016/0306-3623(85)90001-1. [DOI] [PubMed] [Google Scholar]
- Cockcroft S., Gomperts B. D. The ATP4- receptor of rat mast cells. Biochem J. 1980 Jun 15;188(3):789–798. doi: 10.1042/bj1880789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowen D. S., Sanders M., Dubyak G. P2-purinergic receptors activate a guanine nucleotide-dependent phospholipase C in membranes from HL-60 cells. Biochim Biophys Acta. 1990 Jul 12;1053(2-3):195–203. doi: 10.1016/0167-4889(90)90014-5. [DOI] [PubMed] [Google Scholar]
- Ehrlich Y. H., Snider R. M., Kornecki E., Garfield M. G., Lenox R. H. Modulation of neuronal signal transduction systems by extracellular ATP. J Neurochem. 1988 Jan;50(1):295–301. doi: 10.1111/j.1471-4159.1988.tb13263.x. [DOI] [PubMed] [Google Scholar]
- Felder C. C., Williams H. L., Axelrod J. A transduction pathway associated with receptors coupled to the inhibitory guanine nucleotide binding protein Gi that amplifies ATP-mediated arachidonic acid release. Proc Natl Acad Sci U S A. 1991 Aug 1;88(15):6477–6480. doi: 10.1073/pnas.88.15.6477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fine J., Cole P., Davidson J. S. Extracellular nucleotides stimulate receptor-mediated calcium mobilization and inositol phosphate production in human fibroblasts. Biochem J. 1989 Oct 15;263(2):371–376. doi: 10.1042/bj2630371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon J. L. Extracellular ATP: effects, sources and fate. Biochem J. 1986 Jan 15;233(2):309–319. doi: 10.1042/bj2330309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grynkiewicz G., Poenie M., Tsien R. Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985 Mar 25;260(6):3440–3450. [PubMed] [Google Scholar]
- Hallam T. J., Pearson J. D. Exogenous ATP raises cytoplasmic free calcium in fura-2 loaded piglet aortic endothelial cells. FEBS Lett. 1986 Oct 20;207(1):95–99. doi: 10.1016/0014-5793(86)80019-9. [DOI] [PubMed] [Google Scholar]
- Iredale P. A., Martin K. F., Alexander S. P., Hill S. J., Kendall D. A. Inositol 1,4,5-trisphosphate generation and calcium mobilisation via activation of an atypical P2 receptor in the neuronal cell line, N1E-115. Br J Pharmacol. 1992 Dec;107(4):1083–1087. doi: 10.1111/j.1476-5381.1992.tb13410.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iredale P. A., Martin K. F., Hill S. J., Kendall D. A. Agonist-induced changes in [Ca2+]i in N1E-115 cells: differential effects of bradykinin and carbachol. Eur J Pharmacol. 1992 Jun 5;226(2):163–168. doi: 10.1016/0922-4106(92)90178-x. [DOI] [PubMed] [Google Scholar]
- Iredale P. A., Martin K. F., Hill S. J., Kendall D. A. The effects of beta-phorbol-12,13 dibutyrate on agonist-induced increases in [Ca2+]i in N1E-115 cells. Differential modulation of responses to angiotensin II and bradykinin. Biochem Pharmacol. 1993 Feb 9;45(3):611–617. doi: 10.1016/0006-2952(93)90134-i. [DOI] [PubMed] [Google Scholar]
- Jacob R. Agonist-stimulated divalent cation entry into single cultured human umbilical vein endothelial cells. J Physiol. 1990 Feb;421:55–77. doi: 10.1113/jphysiol.1990.sp017933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kastritsis C. H., Salm A. K., McCarthy K. Stimulation of the P2Y purinergic receptor on type 1 astroglia results in inositol phosphate formation and calcium mobilization. J Neurochem. 1992 Apr;58(4):1277–1284. doi: 10.1111/j.1471-4159.1992.tb11339.x. [DOI] [PubMed] [Google Scholar]
- Koike M., Kashiwagura T., Takeguchi N. Gluconeogenesis stimulated by extracellular ATP is triggered by the initial increase in the intracellular Ca2+ concentration of the periphery of hepatocytes. Biochem J. 1992 Apr 1;283(Pt 1):265–272. doi: 10.1042/bj2830265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lustig K. D., Sportiello M. G., Erb L., Weisman G. A. A nucleotide receptor in vascular endothelial cells is specifically activated by the fully ionized forms of ATP and UTP. Biochem J. 1992 Jun 15;284(Pt 3):733–739. doi: 10.1042/bj2840733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murrin R. J., Boarder M. R. Neuronal "nucleotide" receptor linked to phospholipase C and phospholipase D? Stimulation of PC12 cells by ATP analogues and UTP. Mol Pharmacol. 1992 Mar;41(3):561–568. [PubMed] [Google Scholar]
- Nishizuka Y. Studies and perspectives of protein kinase C. Science. 1986 Jul 18;233(4761):305–312. doi: 10.1126/science.3014651. [DOI] [PubMed] [Google Scholar]
- O'Connor S. E. Recent developments in the classification and functional significance of receptors for ATP and UTP, evidence for nucleotide receptors. Life Sci. 1992;50(22):1657–1664. doi: 10.1016/0024-3205(92)90420-t. [DOI] [PubMed] [Google Scholar]
- Pavenstädt H., Späth M., Schlunck G., Nauck M., Fischer R., Wanner C., Schollmeyer P. Effect of nucleotides on the cytosolic free calcium activity and inositol phosphate formation in human glomerular epithelial cells. Br J Pharmacol. 1992 Sep;107(1):189–195. doi: 10.1111/j.1476-5381.1992.tb14485.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeilschifter J. Comparison of extracellular ATP and UTP signalling in rat renal mesangial cells. No indications for the involvement of separate purino- and pyrimidino-ceptors. Biochem J. 1990 Dec 1;272(2):469–472. doi: 10.1042/bj2720469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phaneuf S., Berta P., Casanova J., Cavadore J. C. ATP stimulates inositol phosphates accumulation and calcium mobilization in a primary culture of rat aortic myocytes. Biochem Biophys Res Commun. 1987 Mar 13;143(2):454–460. doi: 10.1016/0006-291x(87)91375-1. [DOI] [PubMed] [Google Scholar]
- Rice W. R., Singleton F. M. P2Y-purinoceptor regulation of surfactant secretion from rat isolated alveolar type II cells is associated with mobilization of intracellular calcium. Br J Pharmacol. 1987 Aug;91(4):833–838. doi: 10.1111/j.1476-5381.1987.tb11282.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato K., Okajima F., Kondo Y. Extracellular ATP stimulates three different receptor-signal transduction systems in FRTL-5 thyroid cells. Activation of phospholipase C, and inhibition and activation of adenylate cyclase. Biochem J. 1992 Apr 1;283(Pt 1):281–287. doi: 10.1042/bj2830281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stutchfield J., Cockcroft S. Undifferentiated HL60 cells respond to extracellular ATP and UTP by stimulating phospholipase C activation and exocytosis. FEBS Lett. 1990 Mar 26;262(2):256–258. doi: 10.1016/0014-5793(90)80204-v. [DOI] [PubMed] [Google Scholar]
- Traiffort E., Ruat M., Arrang J. M., Leurs R., Piomelli D., Schwartz J. C. Expression of a cloned rat histamine H2 receptor mediating inhibition of arachidonate release and activation of cAMP accumulation. Proc Natl Acad Sci U S A. 1992 Apr 1;89(7):2649–2653. doi: 10.1073/pnas.89.7.2649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Kügelgen I., Bültmann R., Starke K. Interaction of adenine nucleotides, UTP and suramin in mouse vas deferens: suramin-sensitive and suramin-insensitive components in the contractile effect of ATP. Naunyn Schmiedebergs Arch Pharmacol. 1990 Aug;342(2):198–205. doi: 10.1007/BF00166965. [DOI] [PubMed] [Google Scholar]


