Abstract
1. Endothelin (ET)-1 has been demonstrated to cause contraction of uterine smooth muscle. We investigated the role of ET receptor subtypes (ETA and ETB receptors) in ET-1-induced contraction of rat uterine smooth muscle by using the ETA receptor antagonist BQ-123 and the ETB receptor agonist BQ-3020. 2. ET-1 caused a contraction with superimposed oscillations of the rat isolated uterus suspended in Krebs-Ringer solution; both the amplitude of contraction as well as the oscillation frequency increased in a dose-dependent manner (10(-11)-10(-7)M). 3. BQ-123 (10(-6)M) markedly shifted the dose-response curve of ET-1 for both contractile effects and oscillation frequency to the right. 4. BQ-3020 (10(-11)-3 x 10(-7) M) did not cause uterine contraction; neither did it affect the dose-response curve of ET-1 for either the contractile effect or the increase in oscillation frequency. Thus, stimulation of ETB receptors is not involved in these responses. 5. The present findings suggest that ET-1-induced contractile responses and the increase in oscillation frequency in rat uterine smooth muscle is mediated through ETA receptors, and that ETB receptors play no role in these responses.
Full text
PDF
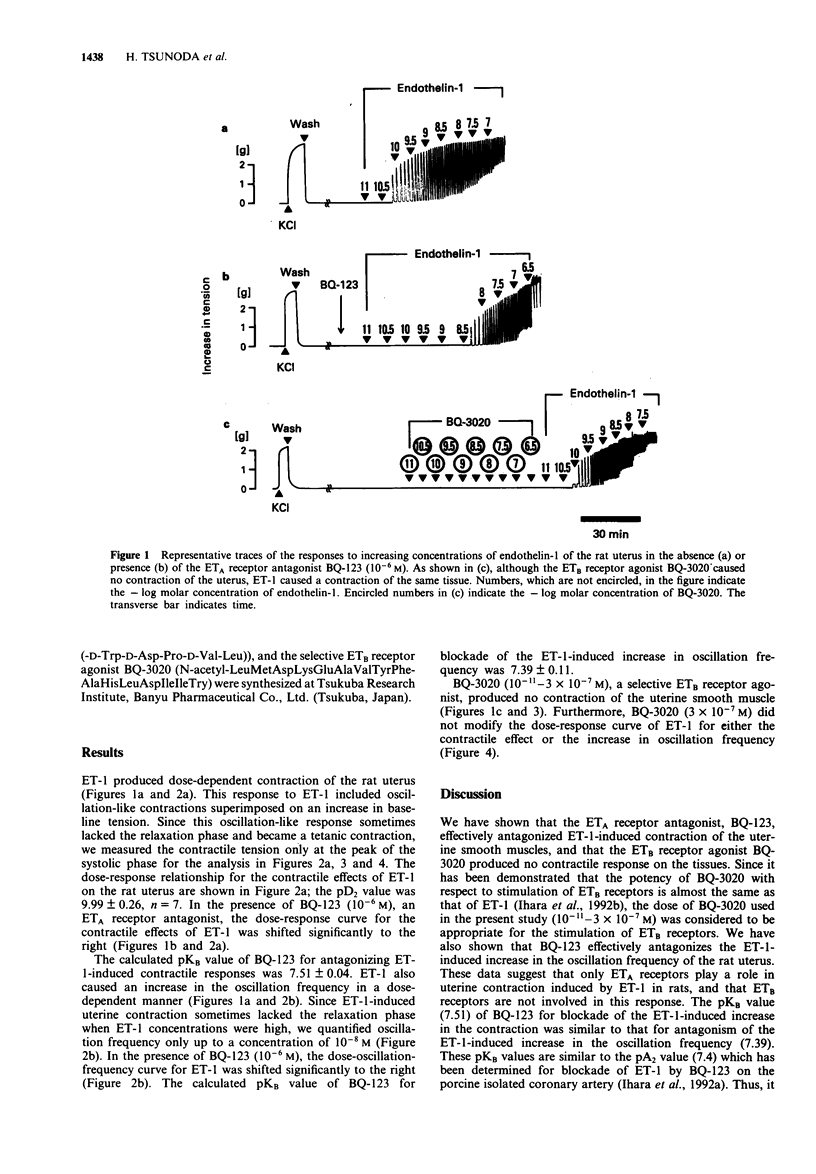
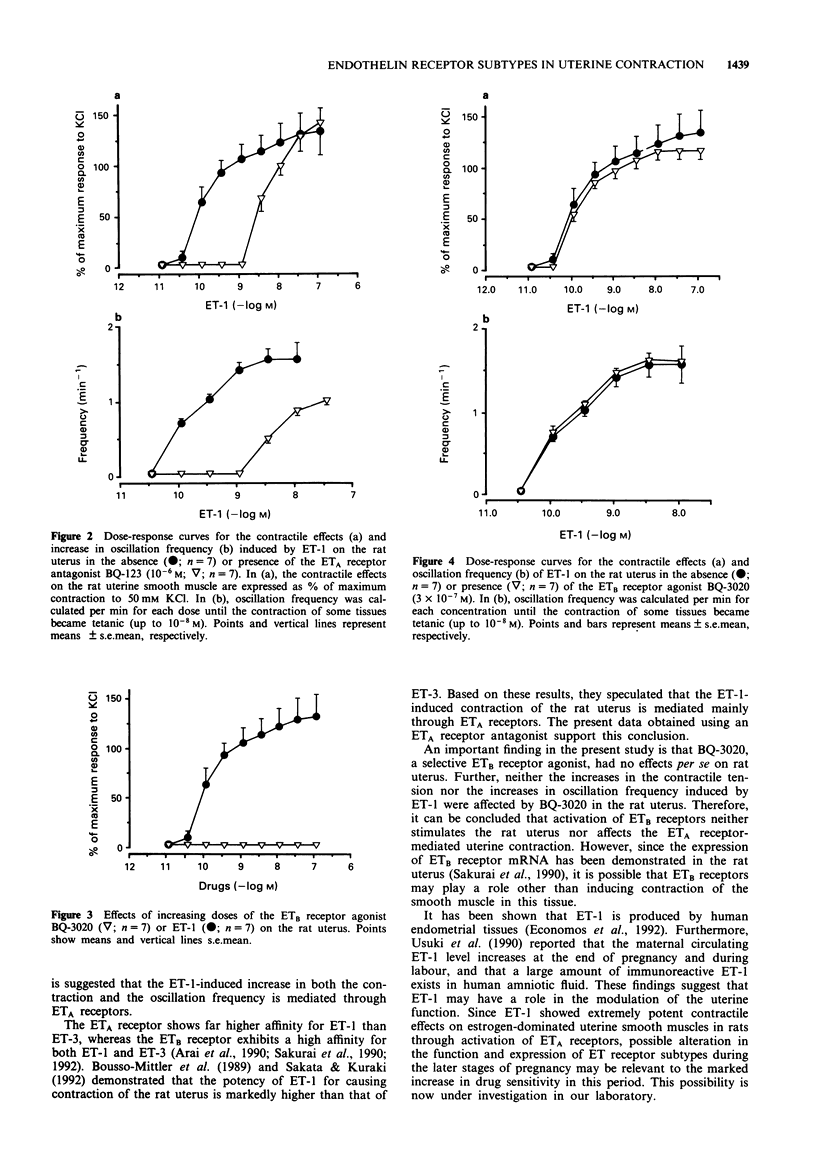

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arai H., Hori S., Aramori I., Ohkubo H., Nakanishi S. Cloning and expression of a cDNA encoding an endothelin receptor. Nature. 1990 Dec 20;348(6303):730–732. doi: 10.1038/348730a0. [DOI] [PubMed] [Google Scholar]
- Bousso-Mittler D., Kloog Y., Wollberg Z., Bdolah A., Kochva E., Sokolovsky M. Functional endothelin/sarafotoxin receptors in the rat uterus. Biochem Biophys Res Commun. 1989 Aug 15;162(3):952–957. doi: 10.1016/0006-291x(89)90765-1. [DOI] [PubMed] [Google Scholar]
- Economos K., MacDonald P. C., Casey M. L. Endothelin-1 gene expression and protein biosynthesis in human endometrium: potential modulator of endometrial blood flow. J Clin Endocrinol Metab. 1992 Jan;74(1):14–19. doi: 10.1210/jcem.74.1.1727813. [DOI] [PubMed] [Google Scholar]
- Ihara M., Noguchi K., Saeki T., Fukuroda T., Tsuchida S., Kimura S., Fukami T., Ishikawa K., Nishikibe M., Yano M. Biological profiles of highly potent novel endothelin antagonists selective for the ETA receptor. Life Sci. 1992;50(4):247–255. doi: 10.1016/0024-3205(92)90331-i. [DOI] [PubMed] [Google Scholar]
- Sakata K., Karaki H. Effects of endothelin on cytosolic Ca2+ level and mechanical activity in rat uterine smooth muscle. Eur J Pharmacol. 1992 Oct 6;221(1):9–15. doi: 10.1016/0014-2999(92)90766-w. [DOI] [PubMed] [Google Scholar]
- Sakurai T., Yanagisawa M., Masaki T. Molecular characterization of endothelin receptors. Trends Pharmacol Sci. 1992 Mar;13(3):103–108. doi: 10.1016/0165-6147(92)90038-8. [DOI] [PubMed] [Google Scholar]
- Sakurai T., Yanagisawa M., Takuwa Y., Miyazaki H., Kimura S., Goto K., Masaki T. Cloning of a cDNA encoding a non-isopeptide-selective subtype of the endothelin receptor. Nature. 1990 Dec 20;348(6303):732–735. doi: 10.1038/348732a0. [DOI] [PubMed] [Google Scholar]
- Takai M., Umemura I., Yamasaki K., Watakabe T., Fujitani Y., Oda K., Urade Y., Inui T., Yamamura T., Okada T. A potent and specific agonist, Suc-[Glu9,Ala11,15]-endothelin-1(8-21), IRL 1620, for the ETB receptor. Biochem Biophys Res Commun. 1992 Apr 30;184(2):953–959. doi: 10.1016/0006-291x(92)90683-c. [DOI] [PubMed] [Google Scholar]
- Usuki S., Saitoh T., Sawamura T., Suzuki N., Shigemitsu S., Yanagisawa M., Goto K., Onda H., Fujino M., Masaki T. Increased maternal plasma concentration of endothelin-1 during labor pain or on delivery and the existence of a large amount of endothelin-1 in amniotic fluid. Gynecol Endocrinol. 1990 Jun;4(2):85–97. doi: 10.3109/09513599009012325. [DOI] [PubMed] [Google Scholar]
- Yanagisawa M., Masaki T. Molecular biology and biochemistry of the endothelins. Trends Pharmacol Sci. 1989 Sep;10(9):374–378. doi: 10.1016/0165-6147(89)90011-4. [DOI] [PubMed] [Google Scholar]


