Abstract
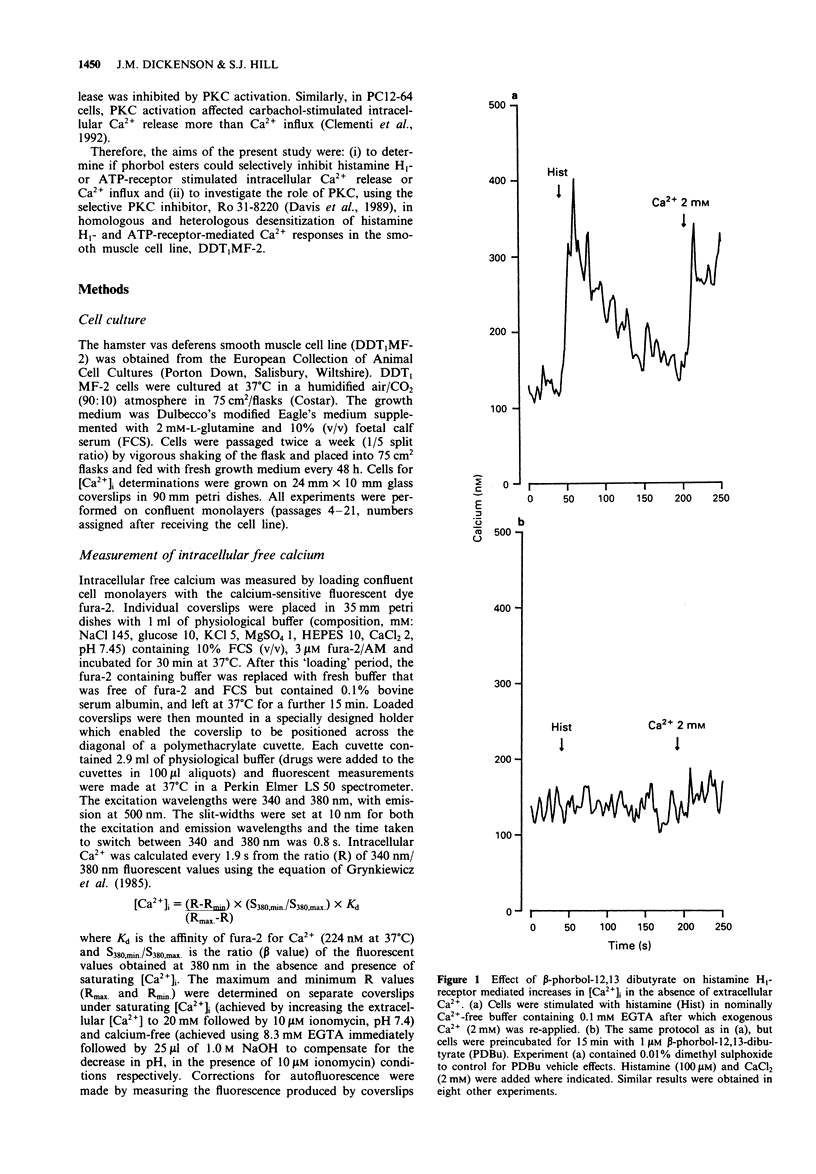
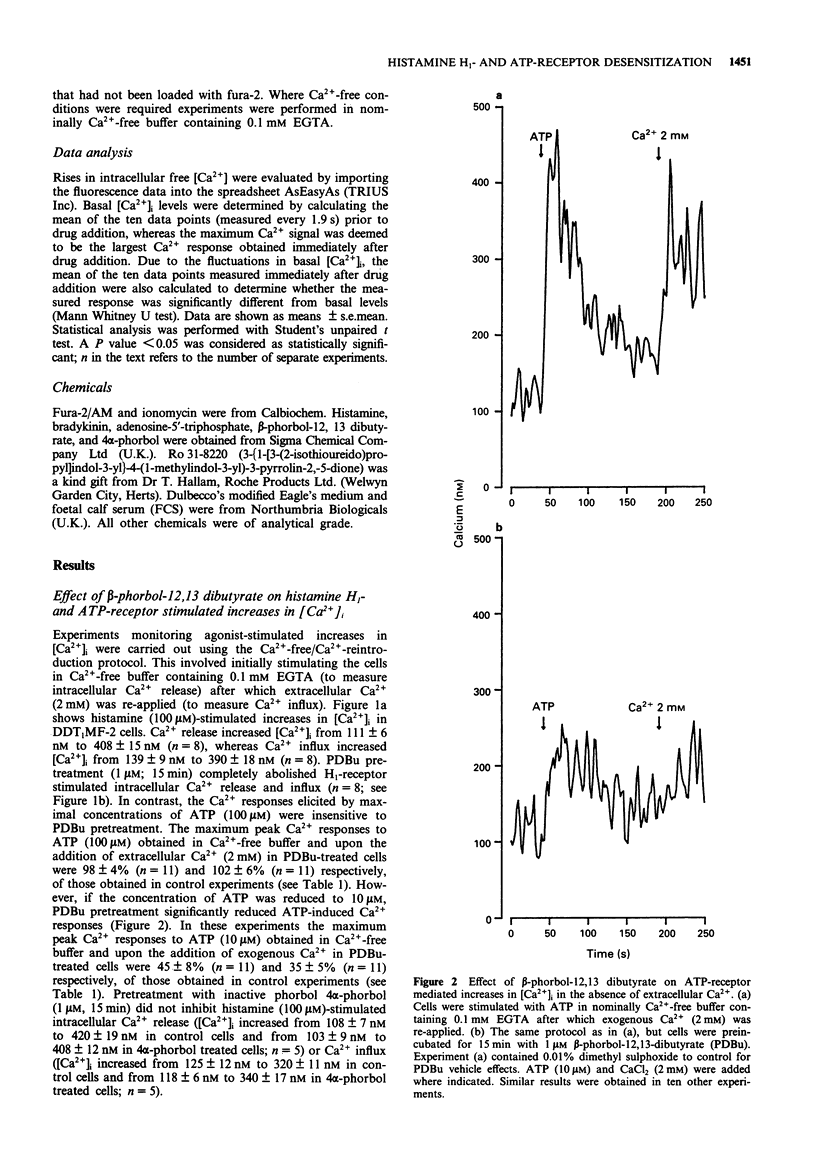
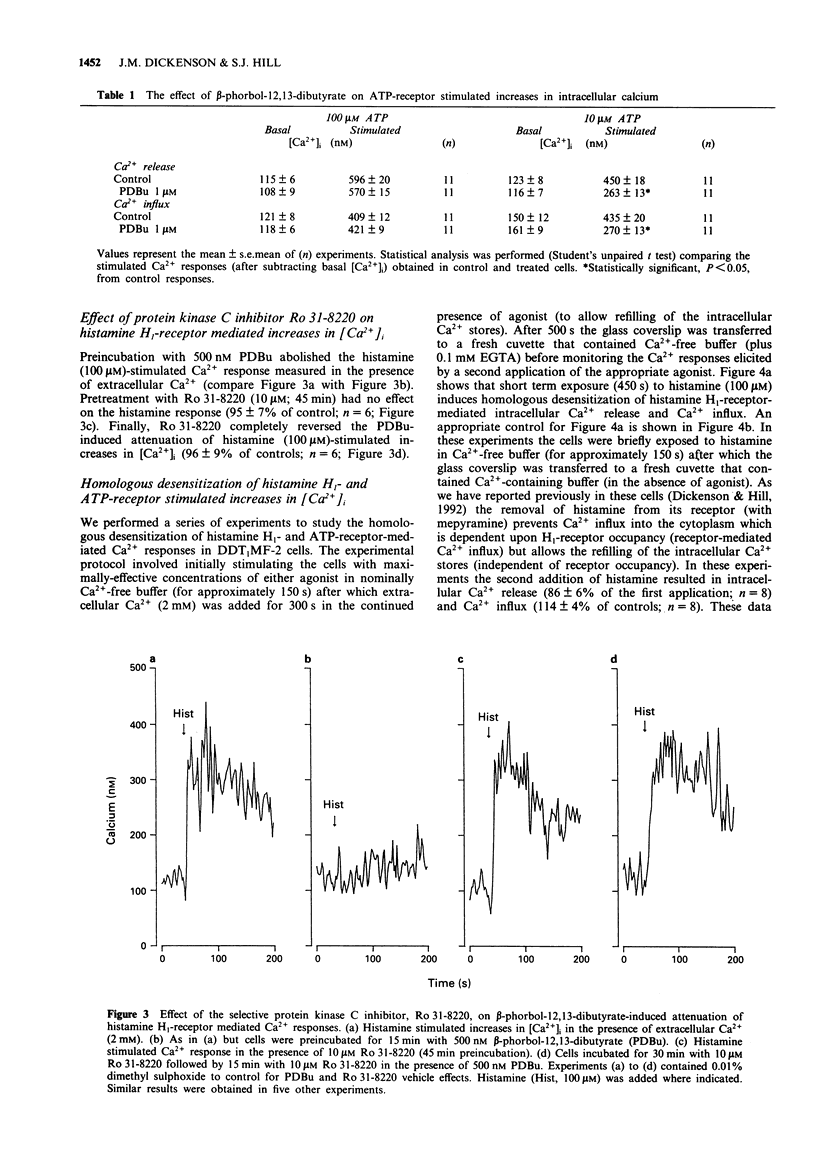
1. The possible role of protein kinase C (PKC) in homologous and heterologous desensitization of histamine H1- and ATP-receptors has been studied in monolayers of cultured vas deferens smooth muscle cells (DDT1MF-2). Cells were loaded with the calcium-sensitive fluorescent dye fura-2 and increases in intracellular free Ca2+ concentration ([Ca2+]i) monitored in response to histamine H1- or ATP-receptor activation. 2. Histamine and ATP stimulated the release of Ca2+ from intracellular Ca2+ stores and Ca2+ influx across the plasma membrane. Activation of PKC with the phorbol ester beta-phorbol-12,13 dibutyrate (PDBu; 1 microM) attenuated histamine (100 microM) and ATP (10 microM)-induced release of intracellular Ca2+ and Ca2+ influx. 3. The selective PKC inhibitor, Ro 31-8220 (10 microM), reversed the PDBu-induced attenuation of histamine (100 microM)-stimulated Ca2+ responses. 4. Histamine H1- and ATP-receptors are readily susceptible to homologous desensitization since short-term exposure to histamine or ATP (450 s) attenuated the Ca2+ responses elicited by a second application of the same agonist. Furthermore, H1-receptor activation-induced heterologous desensitization of ATP stimulated Ca2+ responses and vice versa. 5. Homologous and heterologous desensitization of histamine and ATP Ca2+ responses still occurred in the presence of the PKC inhibitor, Ro 31-8220 (10 microM). 6. These data suggest that PKC activation can attenuate histamine H1- and ATP-receptor mediated Ca2+ responses. However, based on our experimental data, PKC-independent mechanisms appear to be involved in the homologous and heterologous desensitization of histamine H1- and ATP-receptor mediated Ca2+ responses in DDT1MF-2 cells.
Full text
PDF
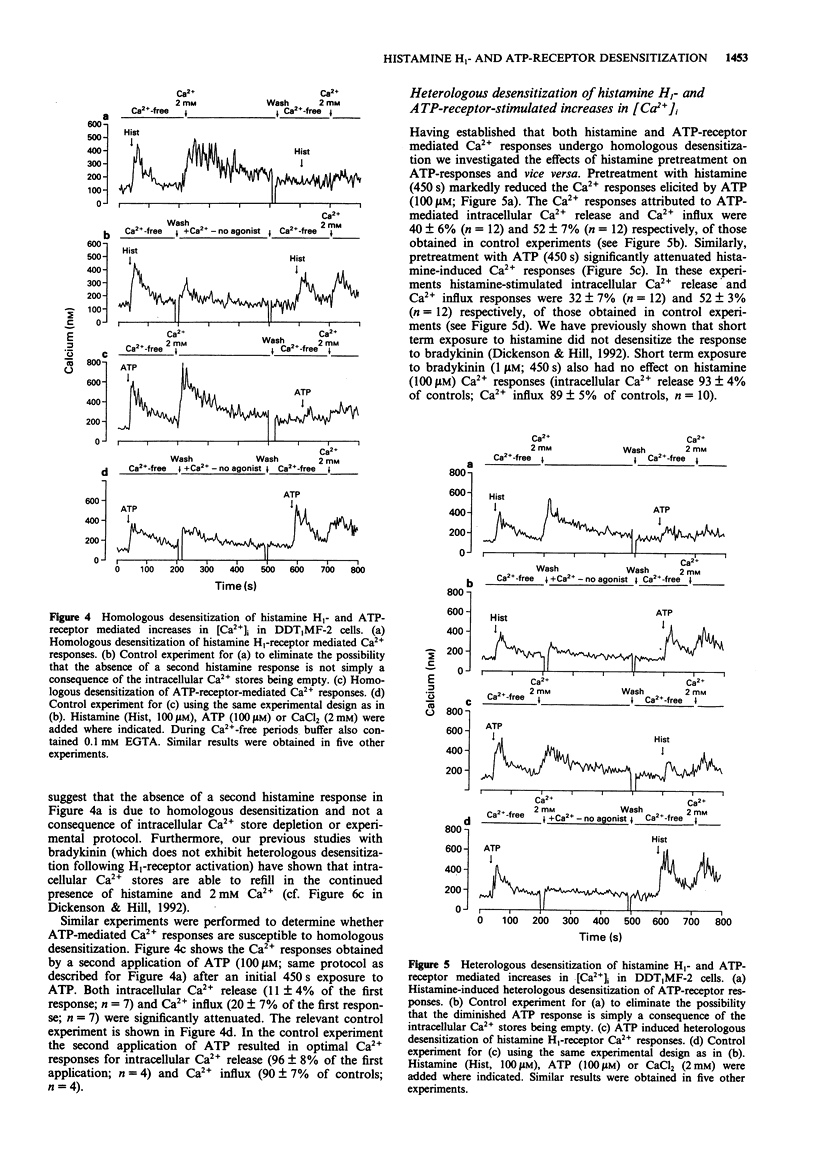


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benovic J. L., Regan J. W., Matsui H., Mayor F., Jr, Cotecchia S., Leeb-Lundberg L. M., Caron M. G., Lefkowitz R. J. Agonist-dependent phosphorylation of the alpha 2-adrenergic receptor by the beta-adrenergic receptor kinase. J Biol Chem. 1987 Dec 25;262(36):17251–17253. [PubMed] [Google Scholar]
- Berridge M. J. Inositol trisphosphate and calcium signalling. Nature. 1993 Jan 28;361(6410):315–325. doi: 10.1038/361315a0. [DOI] [PubMed] [Google Scholar]
- Boarder M. R., Challiss R. A. Role of protein kinase C in the regulation of histamine and bradykinin stimulated inositol polyphosphate turnover in adrenal chromaffin cells. Br J Pharmacol. 1992 Dec;107(4):1140–1145. doi: 10.1111/j.1476-5381.1992.tb13420.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown K. D., Blakeley D. M., Hamon M. H., Laurie M. S., Corps A. N. Protein kinase C-mediated negative-feedback inhibition of unstimulated and bombesin-stimulated polyphosphoinositide hydrolysis in Swiss-mouse 3T3 cells. Biochem J. 1987 Aug 1;245(3):631–639. doi: 10.1042/bj2450631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clementi E., Scheer H., Zacchetti D., Fasolato C., Pozzan T., Meldolesi J. Receptor-activated Ca2+ influx. Two independently regulated mechanisms of influx stimulation coexist in neurosecretory PC12 cells. J Biol Chem. 1992 Feb 5;267(4):2164–2172. [PubMed] [Google Scholar]
- Cowlen M. S., Barnes M. R., Toews M. L. Regulation of histamine H1 receptor-mediated phosphoinositide hydrolysis by histamine and phorbol esters in DDT1 MF-2 cells. Eur J Pharmacol. 1990 Mar 13;188(2-3):105–112. doi: 10.1016/0922-4106(90)90045-y. [DOI] [PubMed] [Google Scholar]
- Cowlen M. S., Toews M. L. Evidence for alpha 1-adrenergic receptor internalization in DDT1 MF-2 cells following exposure to agonists plus protein kinase C activators. Mol Pharmacol. 1988 Sep;34(3):340–346. [PubMed] [Google Scholar]
- Davis P. D., Hill C. H., Keech E., Lawton G., Nixon J. S., Sedgwick A. D., Wadsworth J., Westmacott D., Wilkinson S. E. Potent selective inhibitors of protein kinase C. FEBS Lett. 1989 Dec 18;259(1):61–63. doi: 10.1016/0014-5793(89)81494-2. [DOI] [PubMed] [Google Scholar]
- Dickenson J. M., Hill S. J. Histamine H1-receptor-mediated calcium influx in DDT1MF-2 cells. Biochem J. 1992 Jun 1;284(Pt 2):425–431. doi: 10.1042/bj2840425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickenson J. M., Hill S. J. Histamine-stimulated increases in intracellular calcium in the smooth muscle cell line, DDT1MF-2. Biochem Pharmacol. 1991 Sep 27;42(8):1545–1550. doi: 10.1016/0006-2952(91)90423-3. [DOI] [PubMed] [Google Scholar]
- Dickenson J. M., White T. E., Hill S. J. The effects of elevated cyclic AMP levels on histamine-H1-receptor-stimulated inositol phospholipid hydrolysis and calcium mobilization in the smooth-muscle cell line DDT1MF-2. Biochem J. 1993 Jun 1;292(Pt 2):409–417. doi: 10.1042/bj2920409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felder C. C., Poulter M. O., Wess J. Muscarinic receptor-operated Ca2+ influx in transfected fibroblast cells is independent of inositol phosphates and release of intracellular Ca2+. Proc Natl Acad Sci U S A. 1992 Jan 15;89(2):509–513. doi: 10.1073/pnas.89.2.509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferris C. D., Cameron A. M., Bredt D. S., Huganir R. L., Snyder S. H. Autophosphorylation of inositol 1,4,5-trisphosphate receptors. J Biol Chem. 1992 Apr 5;267(10):7036–7041. [PubMed] [Google Scholar]
- Fujimoto K., Horio Y., Sugama K., Ito S., Liu Y. Q., Fukui H. Genomic cloning of the rat histamine H1 receptor. Biochem Biophys Res Commun. 1993 Jan 15;190(1):294–301. doi: 10.1006/bbrc.1993.1045. [DOI] [PubMed] [Google Scholar]
- Grynkiewicz G., Poenie M., Tsien R. Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985 Mar 25;260(6):3440–3450. [PubMed] [Google Scholar]
- Hausdorff W. P., Caron M. G., Lefkowitz R. J. Turning off the signal: desensitization of beta-adrenergic receptor function. FASEB J. 1990 Aug;4(11):2881–2889. [PubMed] [Google Scholar]
- Jones J. A., Owen P. J., Boarder M. R. Influence of phorbol esters, and diacylglycerol kinase and lipase inhibitors on noradrenaline release and phosphoinositide hydrolysis in chromaffin cells. Br J Pharmacol. 1990 Nov;101(3):521–526. doi: 10.1111/j.1476-5381.1990.tb14114.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwatra M. M., Benovic J. L., Caron M. G., Lefkowitz R. J., Hosey M. M. Phosphorylation of chick heart muscarinic cholinergic receptors by the beta-adrenergic receptor kinase. Biochemistry. 1989 May 30;28(11):4543–4547. doi: 10.1021/bi00437a005. [DOI] [PubMed] [Google Scholar]
- Leeb-Lundberg L. M., Cotecchia S., DeBlasi A., Caron M. G., Lefkowitz R. J. Regulation of adrenergic receptor function by phosphorylation. I. Agonist-promoted desensitization and phosphorylation of alpha 1-adrenergic receptors coupled to inositol phospholipid metabolism in DDT1 MF-2 smooth muscle cells. J Biol Chem. 1987 Mar 5;262(7):3098–3105. [PubMed] [Google Scholar]
- Leeb-Lundberg L. M., Cotecchia S., Lomasney J. W., DeBernardis J. F., Lefkowitz R. J., Caron M. G. Phorbol esters promote alpha 1-adrenergic receptor phosphorylation and receptor uncoupling from inositol phospholipid metabolism. Proc Natl Acad Sci U S A. 1985 Sep;82(17):5651–5655. doi: 10.1073/pnas.82.17.5651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitsuhashi M., Payan D. G. Phorbol ester-mediated desensitization of histamine H1 receptors on a cultured smooth muscle cell line. Life Sci. 1988;43(18):1433–1440. doi: 10.1016/0024-3205(88)90254-8. [DOI] [PubMed] [Google Scholar]
- Palczewski K., Benovic J. L. G-protein-coupled receptor kinases. Trends Biochem Sci. 1991 Oct;16(10):387–391. doi: 10.1016/0968-0004(91)90157-q. [DOI] [PubMed] [Google Scholar]
- Smit M. J., Bloemers S. M., Leurs R., Tertoolen L. G., Bast A., de Laat S. W., Timmerman H. Short-term desensitization of the histamine H1 receptor in human HeLa cells: involvement of protein kinase C dependent and independent pathways. Br J Pharmacol. 1992 Oct;107(2):448–455. doi: 10.1111/j.1476-5381.1992.tb12766.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strasser R. H., Benovic J. L., Caron M. G., Lefkowitz R. J. Beta-agonist- and prostaglandin E1-induced translocation of the beta-adrenergic receptor kinase: evidence that the kinase may act on multiple adenylate cyclase-coupled receptors. Proc Natl Acad Sci U S A. 1986 Sep;83(17):6362–6366. doi: 10.1073/pnas.83.17.6362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods N. M., Cuthbertson K. S., Cobbold P. H. Phorbol-ester-induced alterations of free calcium ion transients in single rat hepatocytes. Biochem J. 1987 Sep 15;246(3):619–623. doi: 10.1042/bj2460619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashita M., Fukui H., Sugama K., Horio Y., Ito S., Mizuguchi H., Wada H. Expression cloning of a cDNA encoding the bovine histamine H1 receptor. Proc Natl Acad Sci U S A. 1991 Dec 15;88(24):11515–11519. doi: 10.1073/pnas.88.24.11515. [DOI] [PMC free article] [PubMed] [Google Scholar]


