Abstract
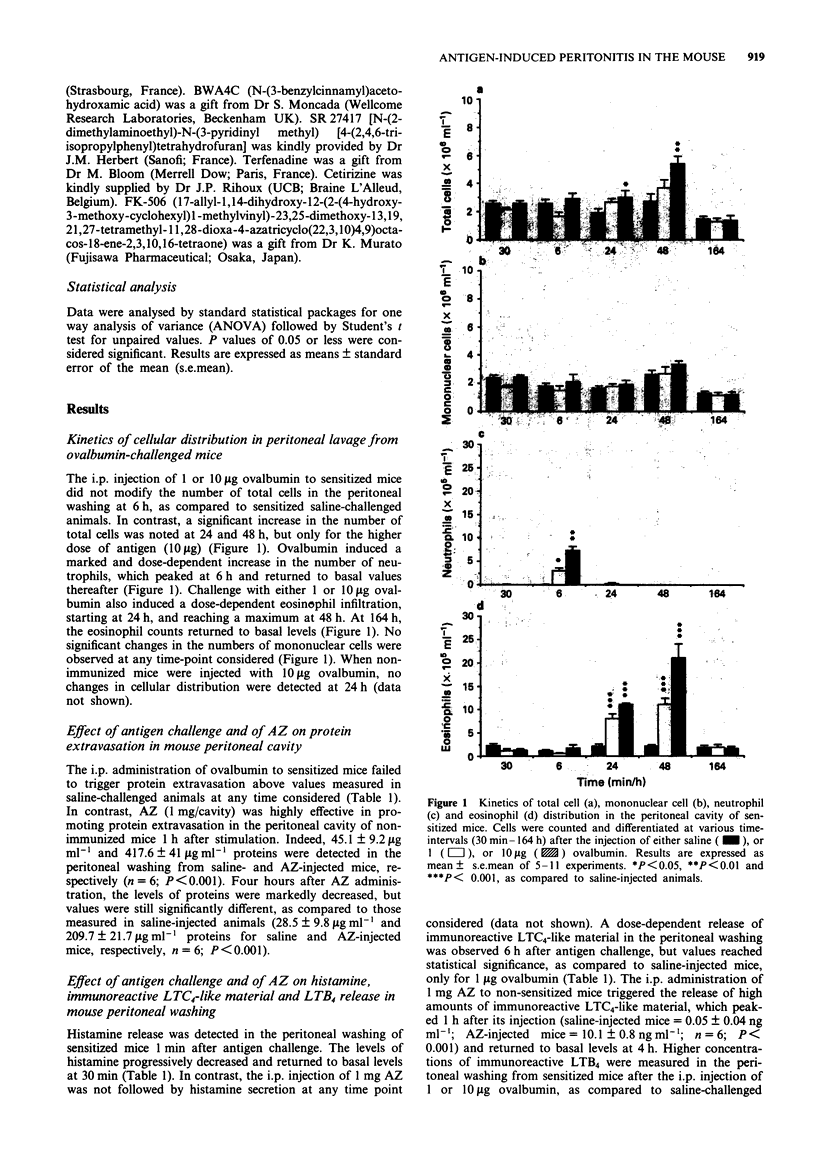
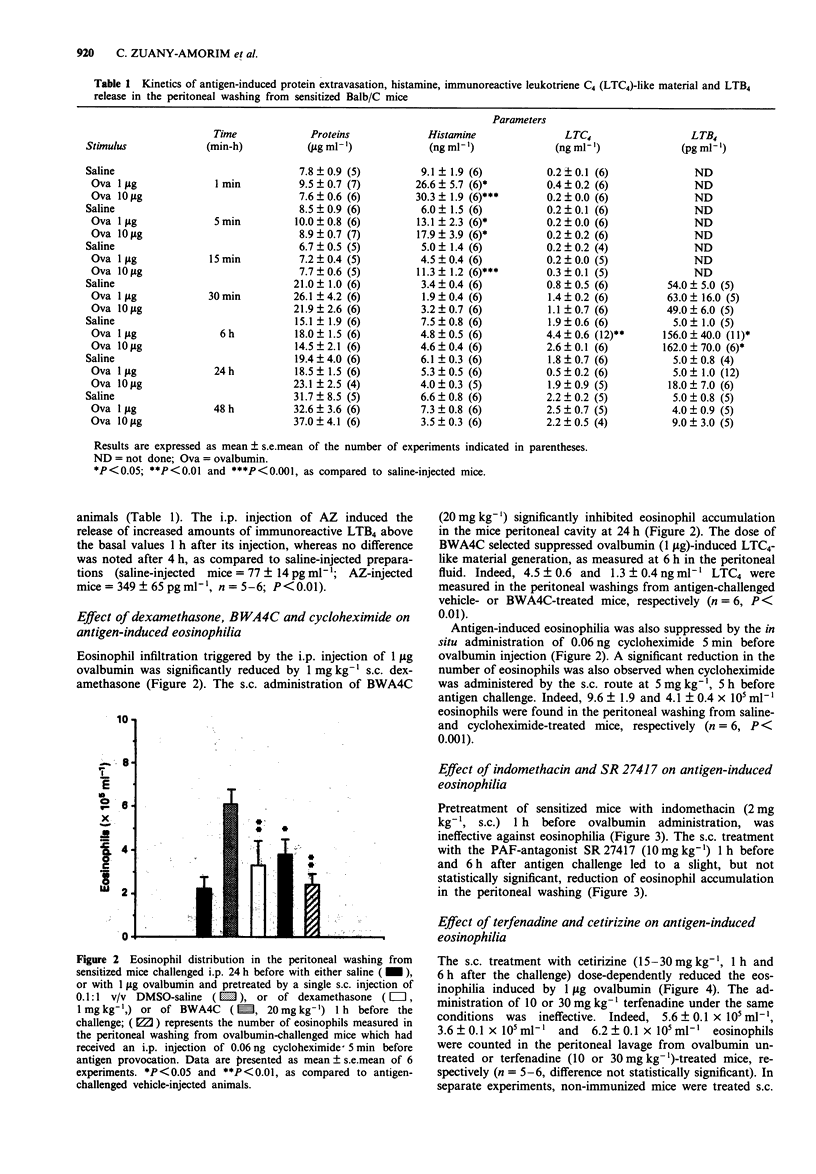
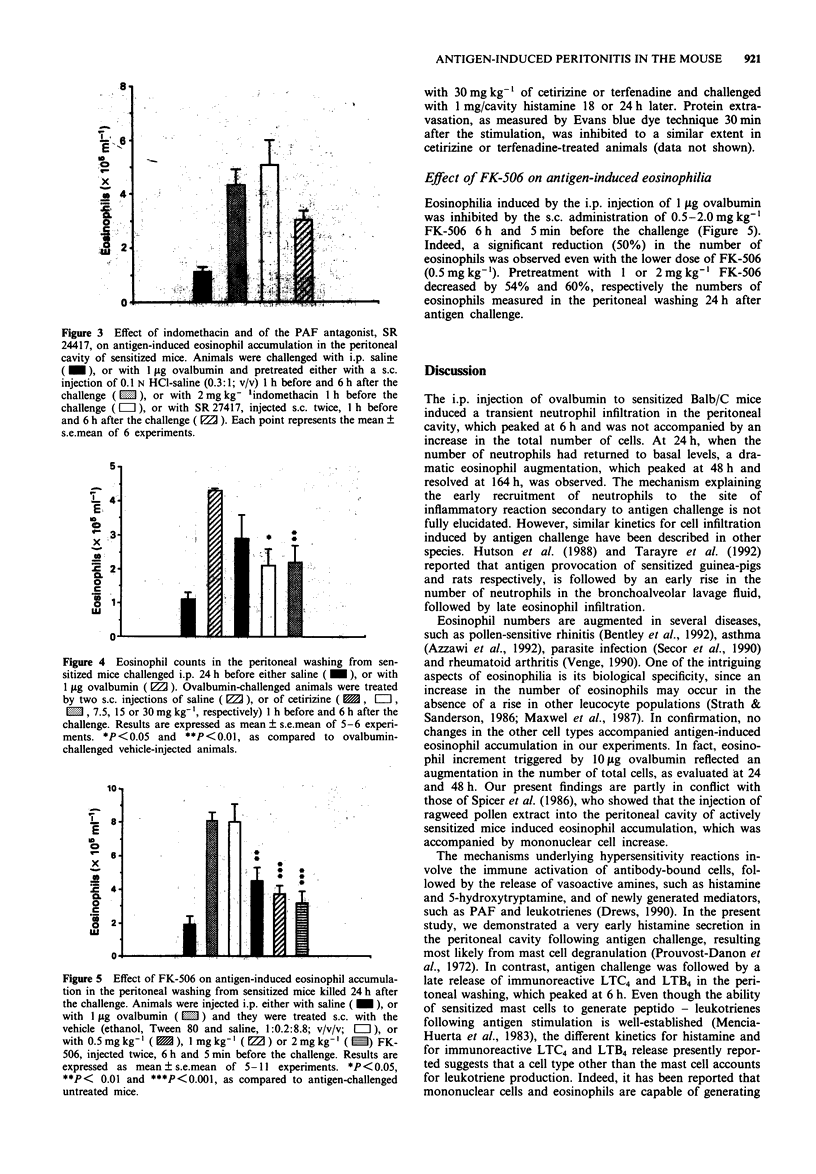
1. The intraperitoneal (i.p.) injection of 1 or 10 micrograms ovalbumin to sensitized Balb/c mice led to an acute histamine release, firstly evidenced 1 min after the challenge and returning to basal levels 30 min thereafter. This phenomenon was unaccompanied by protein extravasation. A dose-dependent increase in the amounts of immunoreactive leukotriene (LT) C4 and LTB4 was observed in the peritoneal washing from sensitized mice 6 h after 1 or 10 micrograms ovalbumin administration. In separate experiments, the i.p. administration of 1 mg activated zymosan to non-immunized mice was followed by a marked protein extravasation, and by immunoreactive LTC4 and LTB4, but not histamine, release in mouse peritoneum 1 h after its injection. 2. Mediator release in the mice peritoneal cavity was concomitant with a transient neutrophil infiltration, which peaked at 6 h and returned to basal levels therefore. An intense eosinophil accumulation starting at 24 h, peaking at 48 h and returning to basal values at 164 h, was also observed. 3. Ovalbumin (1 microgram)-induced eosinophilia, observed at 24 h, was reduced by the pretreatment of the animals with dexamethasone (1 mg kg-1, s.c.) or with the 5-lipoxygenase inhibitor, BWA4C (20 mg kg-1, s.c.), whereas indomethacin (2 mg kg-1, s.c.) and the platelet-activating factor (PAF)-antagonist SR 27417 (10 mg kg-1, s.c.) were ineffective. These results indicate that metabolites of arachidonic acid of lipoxygenase pathway, but not cyclo-oxygenase derivatives or PAF, mediate antigen-induced eosinophil accumulation in the mouse peritoneum.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aehringhaus U., Wölbling R. H., König W., Patrono C., Peskar B. M., Peskar B. A. Release of leukotriene C4 from human polymorphonuclear leucocytes as determined by radioimmunoassay. FEBS Lett. 1982 Sep 6;146(1):111–114. doi: 10.1016/0014-5793(82)80715-1. [DOI] [PubMed] [Google Scholar]
- Amorim C. Z., Cordeiro R. S., Vargaftig B. B. Interference of antihistamines and anti-allergic drugs with antigen-induced paw edema in boosted and unboosted mice. Eur J Pharmacol. 1992 Jun 17;216(3):429–434. doi: 10.1016/0014-2999(92)90441-6. [DOI] [PubMed] [Google Scholar]
- Amorim C. Z., Martins M. A., Cordeiro R. S., Vargaftig B. B. Differential inhibition by the PAF receptor antagonist, WEB 2170, of allergic inflammation in single sensitized and boosted mice. Eur J Pharmacol. 1992 Jan 28;211(1):29–33. doi: 10.1016/0014-2999(92)90257-5. [DOI] [PubMed] [Google Scholar]
- Andersson P., Brattsand R. Protective effects of the glucocorticoid, budesonide, on lung anaphylaxis in actively sensitized guinea-pigs: inhibition of IgE-but not of IgG-mediated anaphylaxis. Br J Pharmacol. 1982 May;76(1):139–147. doi: 10.1111/j.1476-5381.1982.tb09199.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azzawi M., Johnston P. W., Majumdar S., Kay A. B., Jeffery P. K. T lymphocytes and activated eosinophils in airway mucosa in fatal asthma and cystic fibrosis. Am Rev Respir Dis. 1992 Jun;145(6):1477–1482. doi: 10.1164/ajrccm/145.6.1477. [DOI] [PubMed] [Google Scholar]
- Bousquet J., Chanez P., Lacoste J. Y., Barnéon G., Ghavanian N., Enander I., Venge P., Ahlstedt S., Simony-Lafontaine J., Godard P. Eosinophilic inflammation in asthma. N Engl J Med. 1990 Oct 11;323(15):1033–1039. doi: 10.1056/NEJM199010113231505. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Bruijnzeel P. L., Warringa R. A., Kok P. T., Kreukniet J. Inhibition of neutrophil and eosinophil induced chemotaxis by nedocromil sodium and sodium cromoglycate. Br J Pharmacol. 1990 Apr;99(4):798–802. doi: 10.1111/j.1476-5381.1990.tb13009.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruynzeel P. L., Kok P. T., Hamelink M. L., Kijne A. M., Verhagen J. Exclusive leukotriene C4 synthesis by purified human eosinophils induced by opsonized zymosan. FEBS Lett. 1985 Sep 23;189(2):350–354. doi: 10.1016/0014-5793(85)81054-1. [DOI] [PubMed] [Google Scholar]
- Colorado A., Slama J. T., Stavinoha W. B. A new method for measuring auricular inflammation in the mouse. J Pharmacol Methods. 1991 Aug;26(1):73–77. doi: 10.1016/0160-5402(91)90056-b. [DOI] [PubMed] [Google Scholar]
- Coëffier E., Joseph D., Vargaftig B. B. LTB4, a potent chemotactic factor for purified guinea-pig eosinophils: interference of PAF-acether antagonists. Int J Immunopharmacol. 1991;13(2-3):273–280. doi: 10.1016/0192-0561(91)90108-j. [DOI] [PubMed] [Google Scholar]
- Desreumaux P., Janin A., Colombel J. F., Prin L., Plumas J., Emilie D., Torpier G., Capron A., Capron M. Interleukin 5 messenger RNA expression by eosinophils in the intestinal mucosa of patients with coeliac disease. J Exp Med. 1992 Jan 1;175(1):293–296. doi: 10.1084/jem.175.1.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunsky E. H., Atkins P. C., Zweiman B. Histologic responses in human skin test reactions to ragweed. IV. Effects of a single intravenous injection of steroids. J Allergy Clin Immunol. 1977 Feb;59(2):142–146. doi: 10.1016/0091-6749(77)90216-0. [DOI] [PubMed] [Google Scholar]
- Flower R. J. Eleventh Gaddum memorial lecture. Lipocortin and the mechanism of action of the glucocorticoids. Br J Pharmacol. 1988 Aug;94(4):987–1015. doi: 10.1111/j.1476-5381.1988.tb11614.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford-Hutchinson A. W., Bray M. A., Doig M. V., Shipley M. E., Smith M. J. Leukotriene B, a potent chemokinetic and aggregating substance released from polymorphonuclear leukocytes. Nature. 1980 Jul 17;286(5770):264–265. doi: 10.1038/286264a0. [DOI] [PubMed] [Google Scholar]
- Gleich G. J. The eosinophil and bronchial asthma: current understanding. J Allergy Clin Immunol. 1990 Feb;85(2):422–436. doi: 10.1016/0091-6749(90)90151-s. [DOI] [PubMed] [Google Scholar]
- Griswold D. E., Webb E. F., Hillegass L. M. Induction of plasma exudation and inflammatory cell infiltration by leukotriene C4 and leukotriene B4 in mouse peritonitis. Inflammation. 1991 Aug;15(4):251–258. doi: 10.1007/BF00917310. [DOI] [PubMed] [Google Scholar]
- Harding M. W., Galat A., Uehling D. E., Schreiber S. L. A receptor for the immunosuppressant FK506 is a cis-trans peptidyl-prolyl isomerase. Nature. 1989 Oct 26;341(6244):758–760. doi: 10.1038/341758a0. [DOI] [PubMed] [Google Scholar]
- Hatfield S. M., Roehm N. W. Cyclosporine and FK506 inhibition of murine mast cell cytokine production. J Pharmacol Exp Ther. 1992 Feb;260(2):680–688. [PubMed] [Google Scholar]
- Herbert J. M., Bernat A., Valette G., Gigo V., Lale A., LaPlace M. C., Lespy L., Savi P., Maffrand J. P., Le Fur G. Biochemical and pharmacological activities of SR 27417, a highly potent, long-acting platelet-activating factor receptor antagonist. J Pharmacol Exp Ther. 1991 Oct;259(1):44–51. [PubMed] [Google Scholar]
- Hutson P. A., Church M. K., Clay T. P., Miller P., Holgate S. T. Early and late-phase bronchoconstriction after allergen challenge of nonanesthetized guinea pigs. I. The association of disordered airway physiology to leukocyte infiltration. Am Rev Respir Dis. 1988 Mar;137(3):548–557. doi: 10.1164/ajrccm/137.3.548. [DOI] [PubMed] [Google Scholar]
- Kaneko M., Hitoshi Y., Takatsu K., Matsumoto S. Role of interleukin-5 in local accumulation of eosinophils in mouse allergic peritonitis. Int Arch Allergy Appl Immunol. 1991;96(1):41–45. doi: 10.1159/000235532. [DOI] [PubMed] [Google Scholar]
- Kino T., Hatanaka H., Hashimoto M., Nishiyama M., Goto T., Okuhara M., Kohsaka M., Aoki H., Imanaka H. FK-506, a novel immunosuppressant isolated from a Streptomyces. I. Fermentation, isolation, and physico-chemical and biological characteristics. J Antibiot (Tokyo) 1987 Sep;40(9):1249–1255. doi: 10.7164/antibiotics.40.1249. [DOI] [PubMed] [Google Scholar]
- Kyan-Aung U., Hallsworth M., Haskard D., De Vos C., Lee T. H. The effects of cetirizine on the adhesion of human eosinophils and neutrophils to cultured human umbilical vein endothelial cells. J Allergy Clin Immunol. 1992 Aug;90(2):270–272. doi: 10.1016/0091-6749(92)90083-e. [DOI] [PubMed] [Google Scholar]
- Lebel B. A high-sampling-rate automated continuous-flow fluorometric technique for the analysis of nanogram levels of histamine in biological samples. Anal Biochem. 1983 Aug;133(1):16–29. doi: 10.1016/0003-2697(83)90217-8. [DOI] [PubMed] [Google Scholar]
- Lellouch-Tubiana A., Lefort J., Simon M. T., Pfister A., Vargaftig B. B. Eosinophil recruitment into guinea pig lungs after PAF-acether and allergen administration. Modulation by prostacyclin, platelet depletion, and selective antagonists. Am Rev Respir Dis. 1988 Apr;137(4):948–954. doi: 10.1164/ajrccm/137.4.948. [DOI] [PubMed] [Google Scholar]
- Leprevost C., Capron M., De Vos C., Tomassini M., Capron A. Inhibition of eosinophil chemotaxis by a new antiallergic compound (cetirizine). Int Arch Allergy Appl Immunol. 1988;87(1):9–13. doi: 10.1159/000234641. [DOI] [PubMed] [Google Scholar]
- Lima M. C., Martins M. A., Perez S. A., Silva P. M., Cordeiro R. S., Vargaftig B. B. Effect of azelastine on platelet-activating factor and antigen-induced pleurisy in rats. Eur J Pharmacol. 1991 May 17;197(2-3):201–207. doi: 10.1016/0014-2999(91)90522-r. [DOI] [PubMed] [Google Scholar]
- Lopez A. F., Sanderson C. J., Gamble J. R., Campbell H. D., Young I. G., Vadas M. A. Recombinant human interleukin 5 is a selective activator of human eosinophil function. J Exp Med. 1988 Jan 1;167(1):219–224. doi: 10.1084/jem.167.1.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez A. F., To L. B., Yang Y. C., Gamble J. R., Shannon M. F., Burns G. F., Dyson P. G., Juttner C. A., Clark S., Vadas M. A. Stimulation of proliferation, differentiation, and function of human cells by primate interleukin 3. Proc Natl Acad Sci U S A. 1987 May;84(9):2761–2765. doi: 10.1073/pnas.84.9.2761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez A. F., Williamson D. J., Gamble J. R., Begley C. G., Harlan J. M., Klebanoff S. J., Waltersdorph A., Wong G., Clark S. C., Vadas M. A. Recombinant human granulocyte-macrophage colony-stimulating factor stimulates in vitro mature human neutrophil and eosinophil function, surface receptor expression, and survival. J Clin Invest. 1986 Nov;78(5):1220–1228. doi: 10.1172/JCI112705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martins M. A., Etienne A., Soulard C., Domingo M. T., Braquet P. Chemotactic effect of PAF-acether on peritoneal eosinophils from normal rats. Braz J Med Biol Res. 1989;22(9):1151–1154. [PubMed] [Google Scholar]
- Maxwell C., Hussain R., Nutman T. B., Poindexter R. W., Little M. D., Schad G. A., Ottesen E. A. The clinical and immunologic responses of normal human volunteers to low dose hookworm (Necator americanus) infection. Am J Trop Med Hyg. 1987 Jul;37(1):126–134. doi: 10.4269/ajtmh.1987.37.126. [DOI] [PubMed] [Google Scholar]
- Mencia-Huerta J. M., Razin E., Ringel E. W., Corey E. J., Hoover D., Austen K. F., Lewis R. A. Immunologic and ionophore-induced generation of leukotriene B4 from mouse bone marrow-derived mast cells. J Immunol. 1983 Apr;130(4):1885–1890. [PubMed] [Google Scholar]
- Okudaira H., Nogami M., Matsuzaki G., Dohi M., Suko M., Kasuya S., Takatsu K. T-cell-dependent accumulation of eosinophils in the lung and its inhibition by monoclonal anti-interleukin-5. Int Arch Allergy Appl Immunol. 1991;94(1-4):171–173. doi: 10.1159/000235354. [DOI] [PubMed] [Google Scholar]
- Owen W. F., Jr, Rothenberg M. E., Silberstein D. S., Gasson J. C., Stevens R. L., Austen K. F., Soberman R. J. Regulation of human eosinophil viability, density, and function by granulocyte/macrophage colony-stimulating factor in the presence of 3T3 fibroblasts. J Exp Med. 1987 Jul 1;166(1):129–141. doi: 10.1084/jem.166.1.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne A. N., Garland L. G., Lees I. W., Salmon J. A. Selective inhibition of arachidonate 5-lipoxygenase by novel acetohydroxamic acids: effects on bronchial anaphylaxis in anaesthetized guinea-pigs. Br J Pharmacol. 1988 Jun;94(2):540–546. doi: 10.1111/j.1476-5381.1988.tb11558.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perretti M., Solito E., Parente L. Evidence that endogenous interleukin-1 is involved in leukocyte migration in acute experimental inflammation in rats and mice. Agents Actions. 1992 Jan;35(1-2):71–78. doi: 10.1007/BF01990954. [DOI] [PubMed] [Google Scholar]
- Plaut M., Pierce J. H., Watson C. J., Hanley-Hyde J., Nordan R. P., Paul W. E. Mast cell lines produce lymphokines in response to cross-linkage of Fc epsilon RI or to calcium ionophores. Nature. 1989 May 4;339(6219):64–67. doi: 10.1038/339064a0. [DOI] [PubMed] [Google Scholar]
- Pradelles P., Grassi J., Maclouf J. Enzyme immunoassays of eicosanoids using acetylcholine esterase as label: an alternative to radioimmunoassay. Anal Chem. 1985 Jun;57(7):1170–1173. doi: 10.1021/ac00284a003. [DOI] [PubMed] [Google Scholar]
- Prouvost-Danon A., Binaghi R., Rochas S., Boussac-Aron Y. Immunochemical identification of mouse IgE. Immunology. 1972 Oct;23(4):481–491. [PMC free article] [PubMed] [Google Scholar]
- Rankin J. A., Hitchcock M., Merrill W., Bach M. K., Brashler J. R., Askenase P. W. IgE-dependent release of leukotriene C4 from alveolar macrophages. Nature. 1982 May 27;297(5864):329–331. doi: 10.1038/297329a0. [DOI] [PubMed] [Google Scholar]
- Rothenberg M. E., Owen W. F., Jr, Silberstein D. S., Woods J., Soberman R. J., Austen K. F., Stevens R. L. Human eosinophils have prolonged survival, enhanced functional properties, and become hypodense when exposed to human interleukin 3. J Clin Invest. 1988 Jun;81(6):1986–1992. doi: 10.1172/JCI113547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanderson C. J. Interleukin-5, eosinophils, and disease. Blood. 1992 Jun 15;79(12):3101–3109. [PubMed] [Google Scholar]
- Sawada S., Suzuki G., Kawase Y., Takaku F. Novel immunosuppressive agent, FK506. In vitro effects on the cloned T cell activation. J Immunol. 1987 Sep 15;139(6):1797–1803. [PubMed] [Google Scholar]
- Schleimer R. P. Effects of glucocorticosteroids on inflammatory cells relevant to their therapeutic applications in asthma. Am Rev Respir Dis. 1990 Feb;141(2 Pt 2):S59–S69. [PubMed] [Google Scholar]
- Secor W. E., Stewart S. J., Colley D. G. Eosinophils and immune mechanisms. VI. The synergistic combination of granulocyte-macrophage colony-stimulating factor and IL-5 accounts for eosinophil-stimulation promoter activity in Schistosoma mansoni-infected mice. J Immunol. 1990 Feb 15;144(4):1484–1489. [PubMed] [Google Scholar]
- Shaw R. J., Walsh G. M., Cromwell O., Moqbel R., Spry C. J., Kay A. B. Activated human eosinophils generate SRS-A leukotrienes following IgG-dependent stimulation. Nature. 1985 Jul 11;316(6024):150–152. doi: 10.1038/316150a0. [DOI] [PubMed] [Google Scholar]
- Sher A., Coffman R. L., Hieny S., Scott P., Cheever A. W. Interleukin 5 is required for the blood and tissue eosinophilia but not granuloma formation induced by infection with Schistosoma mansoni. Proc Natl Acad Sci U S A. 1990 Jan;87(1):61–65. doi: 10.1073/pnas.87.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siekierka J. J., Hung S. H., Poe M., Lin C. S., Sigal N. H. A cytosolic binding protein for the immunosuppressant FK506 has peptidyl-prolyl isomerase activity but is distinct from cyclophilin. Nature. 1989 Oct 26;341(6244):755–757. doi: 10.1038/341755a0. [DOI] [PubMed] [Google Scholar]
- Spicer B. A., Hatt P. A., Laycock S. M., Smith H. Effect of drugs on the increase in cell numbers in the peritoneal cavity of the actively sensitised mouse after intraperitoneal challenge with antigen. Int Arch Allergy Appl Immunol. 1986;81(1):81–84. doi: 10.1159/000234112. [DOI] [PubMed] [Google Scholar]
- Strath M., Sanderson C. J. Detection of eosinophil differentiation factor and its relationship to eosinophilia in Mesocestoides corti-infected mice. Exp Hematol. 1986 Jan;14(1):16–20. [PubMed] [Google Scholar]
- Tarayre J. P., Aliaga M., Barbara M., Tisseyre N., Vieu S., Tisne-Versailles J. Model of bronchial allergic inflammation in the brown Norway rat. Pharmacological modulation. Int J Immunopharmacol. 1992 Jul;14(5):847–855. doi: 10.1016/0192-0561(92)90083-w. [DOI] [PubMed] [Google Scholar]
- Venge P. The human eosinophil in inflammation. Agents Actions. 1990 Jan;29(1-2):122–126. doi: 10.1007/BF01964739. [DOI] [PubMed] [Google Scholar]
- Yamamoto K., Mori A., Nakahama T., Ito M., Okudaira H., Miyamoto T. Experimental treatment of autoimmune MRL-lpr/lpr mice with immunosuppressive compound FK506. Immunology. 1990 Feb;69(2):222–227. [PMC free article] [PubMed] [Google Scholar]
- Young P. R., Bell R. L., Lanni C., Summers J. B., Brooks D. W., Carter G. W. Inhibition of leukotriene biosynthesis in the rat peritoneal cavity. Eur J Pharmacol. 1991 Dec 3;205(3):259–266. doi: 10.1016/0014-2999(91)90907-8. [DOI] [PubMed] [Google Scholar]
- Zweiman B., Slott R. I., Atkins P. C. Histologic studies of human skin test responses to ragweed and compound 48/80. III. Effects of alternate-day steroid therapy. J Allergy Clin Immunol. 1976 Dec;58(6):657–663. doi: 10.1016/0091-6749(76)90177-9. [DOI] [PubMed] [Google Scholar]


