Abstract
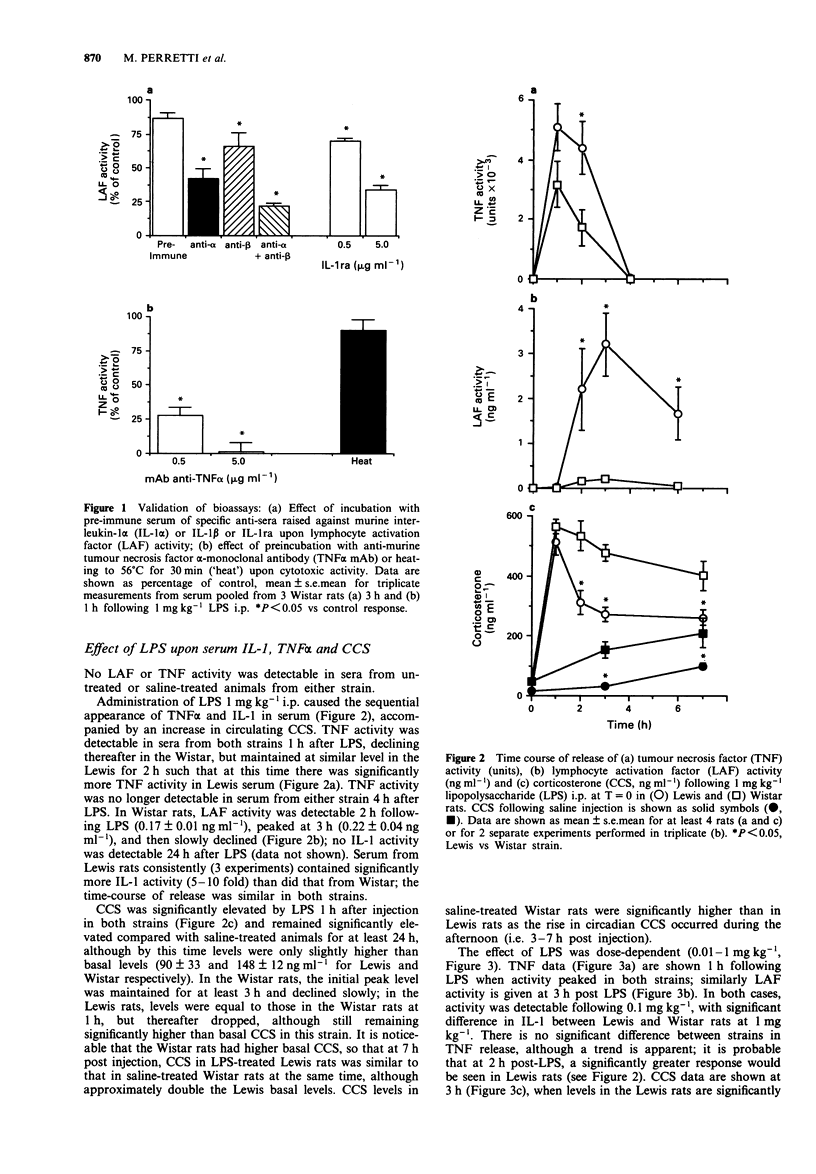
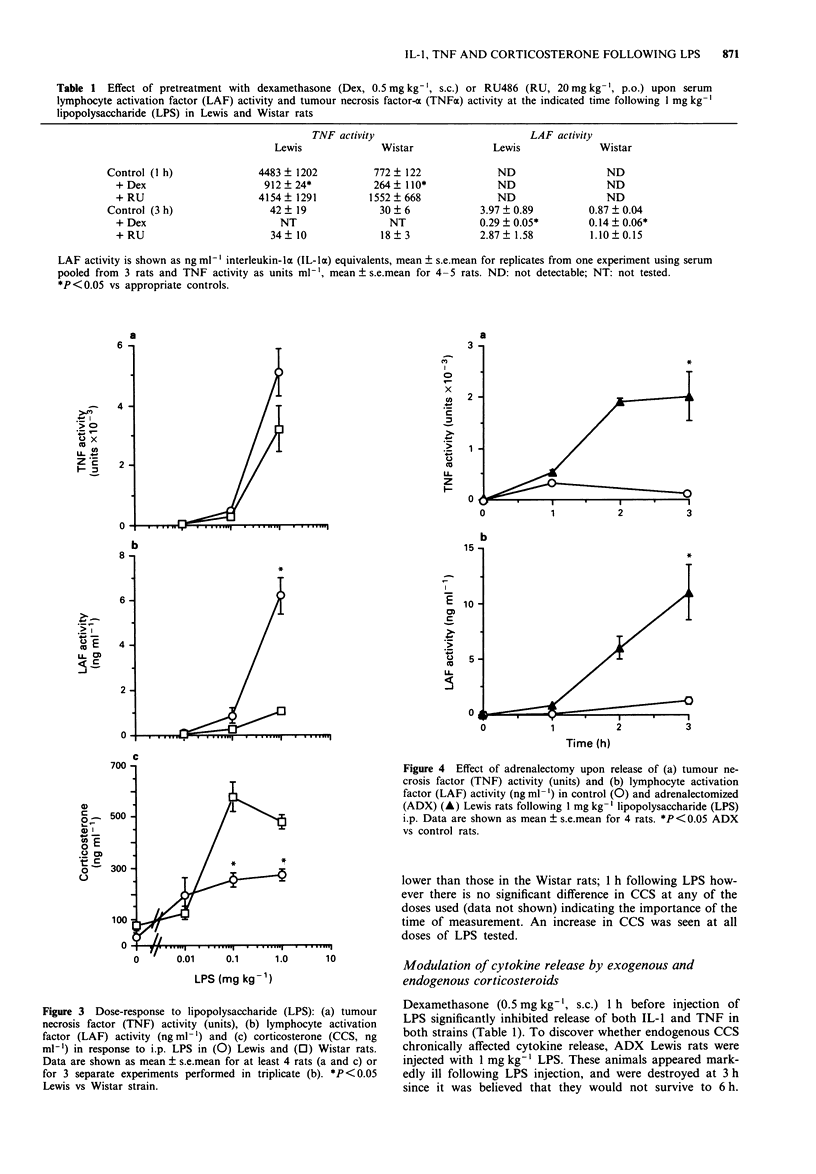
1. Circulating corticosterone, interleukin-1 (IL-1) and tumour necrosis factor-alpha (TNF alpha) activities in serum of Lewis and Wistar rats were measured following injection of lipopolysaccharide (LPS). IL-1 was measured as 'lymphocyte activation factor' (LAF) activity following precipitation of inhibitory activity with polyethylene glycol. TNF alpha activity was measured as cytotoxic activity. 2. Compared to the Wistar, the Lewis rat had higher circulating LAF and TNF activities following LPS, and release of both cytokines was prolonged in this strain. 3. Corticosterone increases in response to LPS were less in the Lewis than in the Wistar rat following the initial peak at 1 h; basal corticosterone was lower in the Lewis rat. 4. Adrenalectomized Lewis rats had even greater amounts of circulating LAF and TNF activities following LPS than did intact animals; the effect of adrenalectomy was not however mimicked by acute treatment with the steroid receptor antagonist, RU486, suggesting that endogenous corticosteroids did not acutely control cytokine release. 5. Although in vivo administration of anti-murine IL-1 alpha antiserum significantly lowered LAF activity of serum, circulating corticosterone in response to LPS was not affected. Similarly, treatment with anti-murine TNF alpha monoclonal antibody (mAb) abrogated TNF activity without affecting corticosterone, suggesting that other mediators may be responsible for corticosterone release following LPS. 6. This 'overproduction' of inflammatory cytokines together with lower circulating corticosterone may contribute to the susceptibility of the Lewis rat to diseases such as adjuvant arthritis or experimental allergic encephalomyelitis.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arend W. P., Dayer J. M. Cytokines and cytokine inhibitors or antagonists in rheumatoid arthritis. Arthritis Rheum. 1990 Mar;33(3):305–315. doi: 10.1002/art.1780330302. [DOI] [PubMed] [Google Scholar]
- Besedovsky H. O., del Rey A., Klusman I., Furukawa H., Monge Arditi G., Kabiersch A. Cytokines as modulators of the hypothalamus-pituitary-adrenal axis. J Steroid Biochem Mol Biol. 1991;40(4-6):613–618. doi: 10.1016/0960-0760(91)90284-c. [DOI] [PubMed] [Google Scholar]
- Beutler B., Krochin N., Milsark I. W., Luedke C., Cerami A. Control of cachectin (tumor necrosis factor) synthesis: mechanisms of endotoxin resistance. Science. 1986 May 23;232(4753):977–980. doi: 10.1126/science.3754653. [DOI] [PubMed] [Google Scholar]
- Bromberg J. S., Chavin K. D., Kunkel S. L. Anti-tumor necrosis factor antibodies suppress cell-mediated immunity in vivo. J Immunol. 1992 Jun 1;148(11):3412–3417. [PubMed] [Google Scholar]
- Calogero A. E., Sternberg E. M., Bagdy G., Smith C., Bernardini R., Aksentijevich S., Wilder R. L., Gold P. W., Chrousos G. P. Neurotransmitter-induced hypothalamic-pituitary-adrenal axis responsiveness is defective in inflammatory disease-susceptible Lewis rats: in vivo and in vitro studies suggesting globally defective hypothalamic secretion of corticotropin-releasing hormone. Neuroendocrinology. 1992 May;55(5):600–608. doi: 10.1159/000126173. [DOI] [PubMed] [Google Scholar]
- Derijk R., Van Rooijen N., Tilders F. J., Besedovsky H. O., Del Rey A., Berkenbosch F. Selective depletion of macrophages prevents pituitary-adrenal activation in response to subpyrogenic, but not to pyrogenic, doses of bacterial endotoxin in rats. Endocrinology. 1991 Jul;129(1):330–338. doi: 10.1210/endo-129-1-330. [DOI] [PubMed] [Google Scholar]
- Dinarello C. A., Cannon J. G., Wolff S. M., Bernheim H. A., Beutler B., Cerami A., Figari I. S., Palladino M. A., Jr, O'Connor J. V. Tumor necrosis factor (cachectin) is an endogenous pyrogen and induces production of interleukin 1. J Exp Med. 1986 Jun 1;163(6):1433–1450. doi: 10.1084/jem.163.6.1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinarello C. A. The proinflammatory cytokines interleukin-1 and tumor necrosis factor and treatment of the septic shock syndrome. J Infect Dis. 1991 Jun;163(6):1177–1184. doi: 10.1093/infdis/163.6.1177. [DOI] [PubMed] [Google Scholar]
- Elenkov I. J., Kovács K., Kiss J., Bertók L., Vizi E. S. Lipopolysaccharide is able to bypass corticotrophin-releasing factor in affecting plasma ACTH and corticosterone levels: evidence from rats with lesions of the paraventricular nucleus. J Endocrinol. 1992 May;133(2):231–236. doi: 10.1677/joe.0.1330231. [DOI] [PubMed] [Google Scholar]
- Evans G. F., Zuckerman S. H. Glucocorticoid-dependent and -independent mechanisms involved in lipopolysaccharide tolerance. Eur J Immunol. 1991 Sep;21(9):1973–1979. doi: 10.1002/eji.1830210902. [DOI] [PubMed] [Google Scholar]
- Flower R. J., Parente L., Persico P., Salmon J. A. A comparison of the acute inflammatory response in adrenalectomised and sham-operated rats. Br J Pharmacol. 1986 Jan;87(1):57–62. doi: 10.1111/j.1476-5381.1986.tb10156.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin A. C., Whitacre C. C. Sex and strain differences in the circadian rhythm fluctuation of endocrine and immune function in the rat: implications for rodent models of autoimmune disease. J Neuroimmunol. 1991 Dec;35(1-3):53–64. doi: 10.1016/0165-5728(91)90161-y. [DOI] [PubMed] [Google Scholar]
- Hawes A. S., Rock C. S., Keogh C. V., Lowry S. F., Calvano S. E. In vivo effects of the antiglucocorticoid RU 486 on glucocorticoid and cytokine responses to Escherichia coli endotoxin. Infect Immun. 1992 Jul;60(7):2641–2647. doi: 10.1128/iai.60.7.2641-2647.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins S. J., Humphreys M. Bioassay of interleukin-1 in serum and plasma following removal of inhibitory activity with polyethylene glycol. J Immunol Methods. 1990 Oct 4;133(1):127–131. doi: 10.1016/0022-1759(90)90326-q. [DOI] [PubMed] [Google Scholar]
- Jacobs C. A., Baker P. E., Roux E. R., Picha K. S., Toivola B., Waugh S., Kennedy M. K. Experimental autoimmune encephalomyelitis is exacerbated by IL-1 alpha and suppressed by soluble IL-1 receptor. J Immunol. 1991 May 1;146(9):2983–2989. [PubMed] [Google Scholar]
- Knudsen P. J., Dinarello C. A., Strom T. B. Glucocorticoids inhibit transcriptional and post-transcriptional expression of interleukin 1 in U937 cells. J Immunol. 1987 Dec 15;139(12):4129–4134. [PubMed] [Google Scholar]
- Lazar G., Jr, Lazar G., Agarwal M. K. Modification of septic shock in mice by the antiglucocorticoid RU 38486. Circ Shock. 1992 Mar;36(3):180–184. [PubMed] [Google Scholar]
- Levine J., Hartwell D., Beller D. I. Imbalanced cytokine production by macrophages from autoimmune-prone mice. Immunol Lett. 1991 Oct;30(2):183–192. doi: 10.1016/0165-2478(91)90023-4. [DOI] [PubMed] [Google Scholar]
- MacPhee I. A., Antoni F. A., Mason D. W. Spontaneous recovery of rats from experimental allergic encephalomyelitis is dependent on regulation of the immune system by endogenous adrenal corticosteroids. J Exp Med. 1989 Feb 1;169(2):431–445. doi: 10.1084/jem.169.2.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason D. Genetic variation in the stress response: susceptibility to experimental allergic encephalomyelitis and implications for human inflammatory disease. Immunol Today. 1991 Feb;12(2):57–60. doi: 10.1016/0167-5699(91)90158-P. [DOI] [PubMed] [Google Scholar]
- McIntyre K. W., Stepan G. J., Kolinsky K. D., Benjamin W. R., Plocinski J. M., Kaffka K. L., Campen C. A., Chizzonite R. A., Kilian P. L. Inhibition of interleukin 1 (IL-1) binding and bioactivity in vitro and modulation of acute inflammation in vivo by IL-1 receptor antagonist and anti-IL-1 receptor monoclonal antibody. J Exp Med. 1991 Apr 1;173(4):931–939. doi: 10.1084/jem.173.4.931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meager A., Leung H., Woolley J. Assays for tumour necrosis factor and related cytokines. J Immunol Methods. 1989 Jan 6;116(1):1–17. doi: 10.1016/0022-1759(89)90306-2. [DOI] [PubMed] [Google Scholar]
- Miller L. C., Dinarello C. A. Biologic activities of interleukin-1 relevant to rheumatic diseases. Pathol Immunopathol Res. 1987;6(1):22–36. doi: 10.1159/000157039. [DOI] [PubMed] [Google Scholar]
- Parant M., Le Contel C., Parant F., Chedid L. Influence of endogenous glucocorticoid on endotoxin-induced production of circulating TNF-alpha. Lymphokine Cytokine Res. 1991 Aug;10(4):265–271. [PubMed] [Google Scholar]
- Peers S. H., Moon D., Flower R. J. Reversal of the anti-inflammatory effects of dexamethasone by the glucocorticoid antagonist RU 38486. Biochem Pharmacol. 1988 Feb 1;37(3):556–557. doi: 10.1016/0006-2952(88)90230-4. [DOI] [PubMed] [Google Scholar]
- Perretti M., Becherucci C., Scapigliati G., Parente L. The effect of adrenalectomy on interleukin-1 release in vitro and in vivo. Br J Pharmacol. 1989 Dec;98(4):1137–1142. doi: 10.1111/j.1476-5381.1989.tb12657.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perretti M., Mugridge K. G., Becherucci C., Parente L. Evidence that interleukin-1 and lipoxygenase metabolites mediate the lethal effect of complete Freund's adjuvant in adrenalectomized rats. Lymphokine Cytokine Res. 1991 Aug;10(4):239–243. [PubMed] [Google Scholar]
- Perretti M., Solito E., Parente L. Evidence that endogenous interleukin-1 is involved in leukocyte migration in acute experimental inflammation in rats and mice. Agents Actions. 1992 Jan;35(1-2):71–78. doi: 10.1007/BF01990954. [DOI] [PubMed] [Google Scholar]
- Rivier C., Chizzonite R., Vale W. In the mouse, the activation of the hypothalamic-pituitary-adrenal axis by a lipopolysaccharide (endotoxin) is mediated through interleukin-1. Endocrinology. 1989 Dec;125(6):2800–2805. doi: 10.1210/endo-125-6-2800. [DOI] [PubMed] [Google Scholar]
- Severn A., Rapson N. T., Hunter C. A., Liew F. Y. Regulation of tumor necrosis factor production by adrenaline and beta-adrenergic agonists. J Immunol. 1992 Jun 1;148(11):3441–3445. [PubMed] [Google Scholar]
- Sternberg E. M., Hill J. M., Chrousos G. P., Kamilaris T., Listwak S. J., Gold P. W., Wilder R. L. Inflammatory mediator-induced hypothalamic-pituitary-adrenal axis activation is defective in streptococcal cell wall arthritis-susceptible Lewis rats. Proc Natl Acad Sci U S A. 1989 Apr;86(7):2374–2378. doi: 10.1073/pnas.86.7.2374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sternberg E. M., Young W. S., 3rd, Bernardini R., Calogero A. E., Chrousos G. P., Gold P. W., Wilder R. L. A central nervous system defect in biosynthesis of corticotropin-releasing hormone is associated with susceptibility to streptococcal cell wall-induced arthritis in Lewis rats. Proc Natl Acad Sci U S A. 1989 Jun;86(12):4771–4775. doi: 10.1073/pnas.86.12.4771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villas P. A., Dronsfield M. J., Blankenhorn E. P. Experimental allergic encephalomyelitis and corticosterone studies in resistant and susceptible rat strains. Clin Immunol Immunopathol. 1991 Oct;61(1):29–40. doi: 10.1016/s0090-1229(06)80005-x. [DOI] [PubMed] [Google Scholar]
- Zuckerman S. H., Evans G. F., Butler L. D. Endotoxin tolerance: independent regulation of interleukin-1 and tumor necrosis factor expression. Infect Immun. 1991 Aug;59(8):2774–2780. doi: 10.1128/iai.59.8.2774-2780.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuckerman S. H., Shellhaas J., Butler L. D. Differential regulation of lipopolysaccharide-induced interleukin 1 and tumor necrosis factor synthesis: effects of endogenous and exogenous glucocorticoids and the role of the pituitary-adrenal axis. Eur J Immunol. 1989 Feb;19(2):301–305. doi: 10.1002/eji.1830190213. [DOI] [PubMed] [Google Scholar]


