Abstract
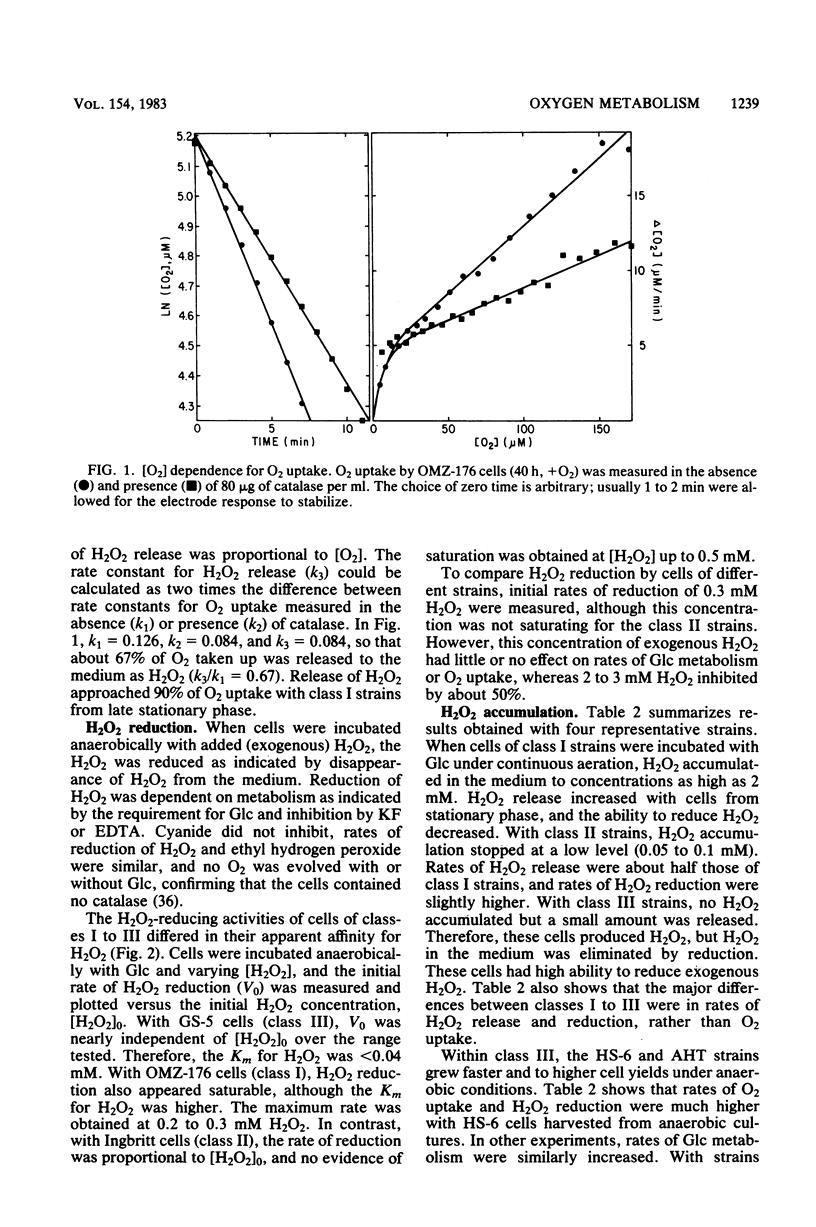
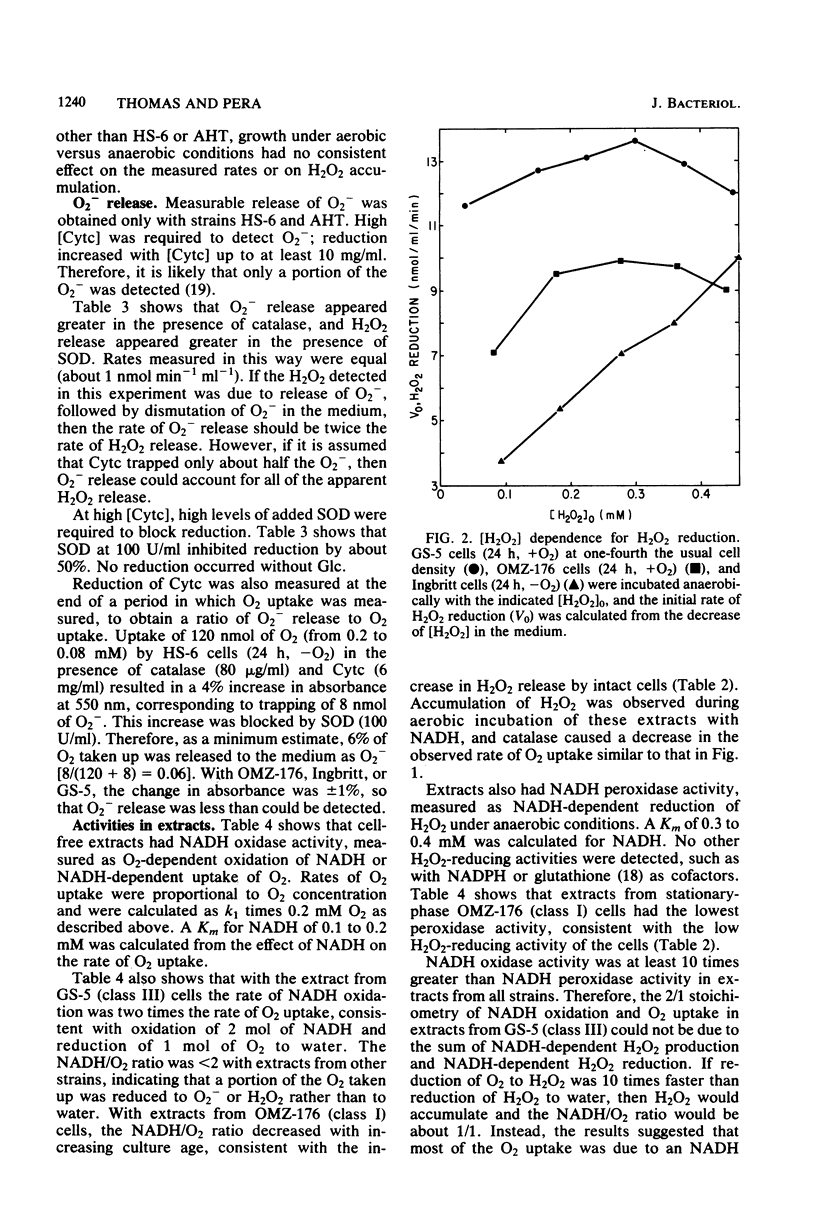
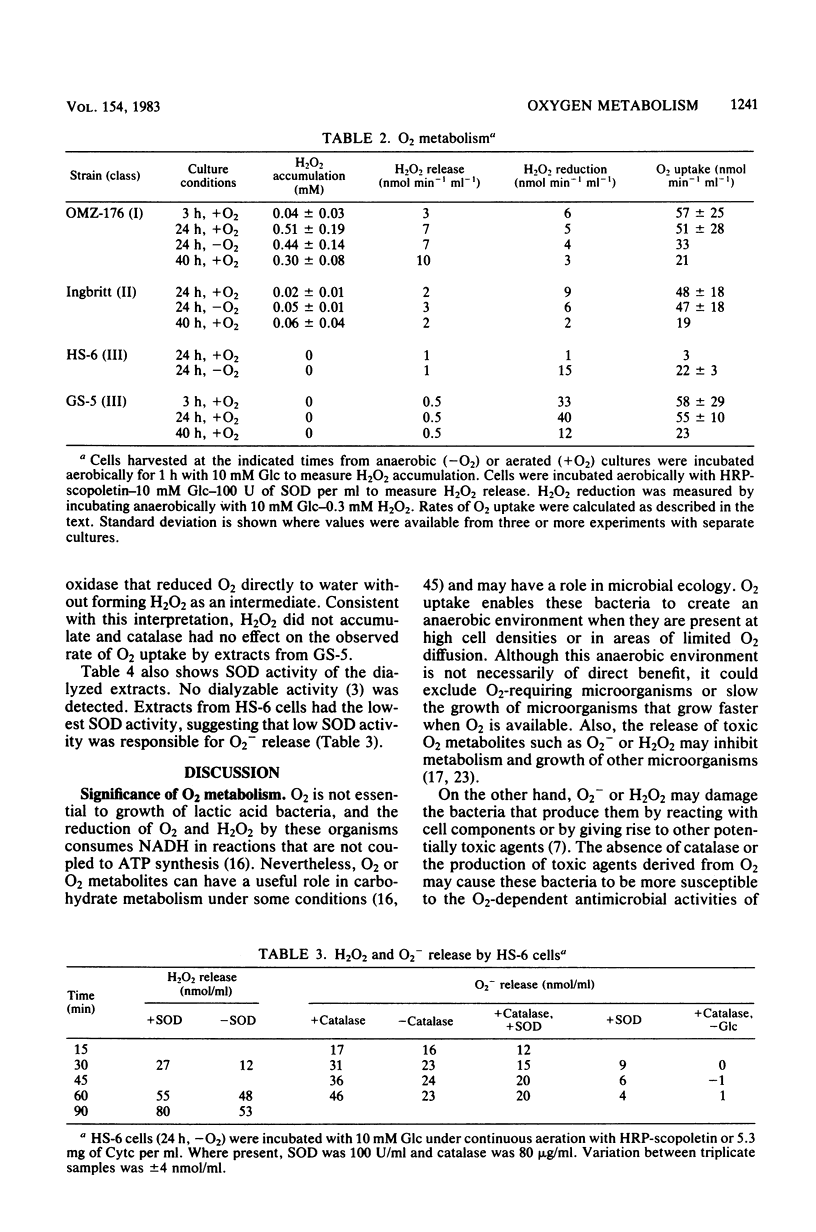
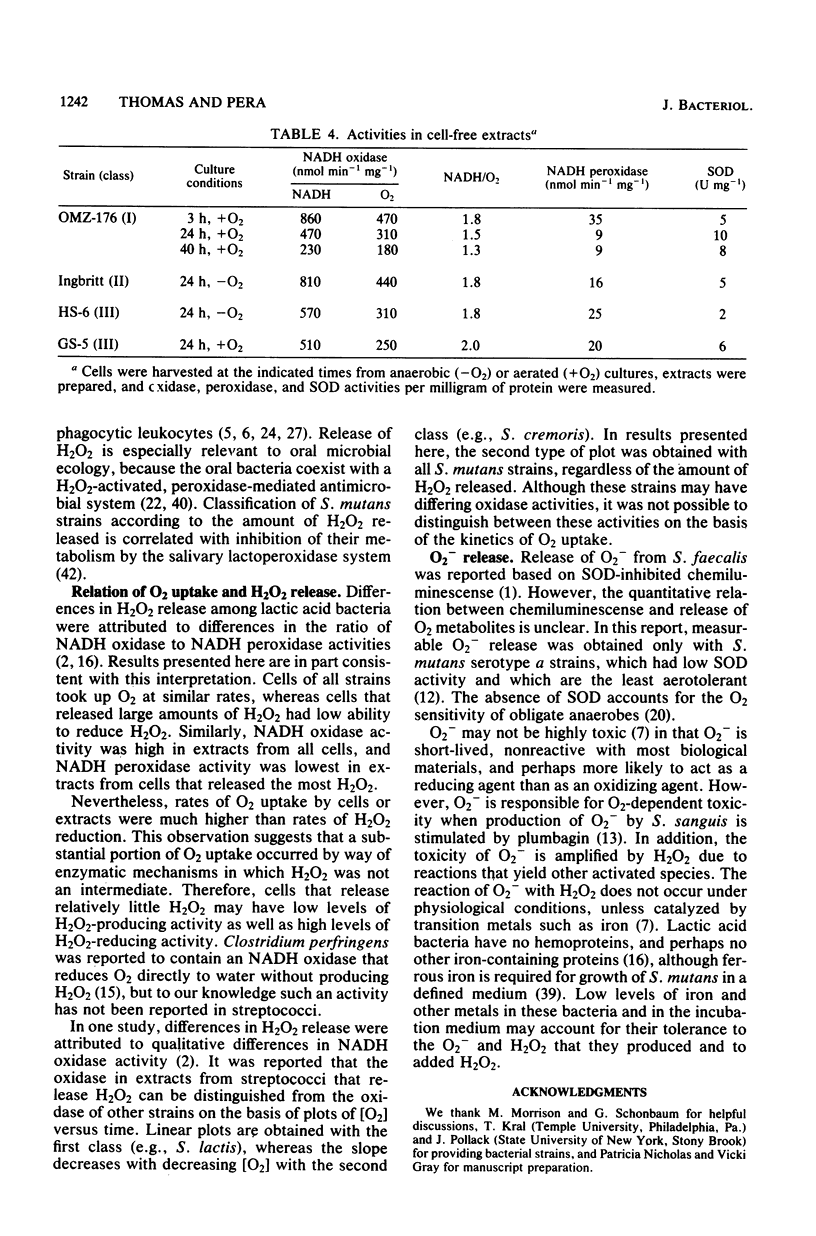
Oxygen (O2) uptake and release of O2 metabolites to the extracellular medium were studied with representatives of serotypes a through g of Streptococcus mutans. When incubated with glucose, washed cells of all strains took up O2 at rates proportional to the O2 concentration. When O2 was held constant at 0.2 mM, 0.2 to 0.5 mol of O2 was taken up per mol of glucose metabolized. Despite the similar rates of O2 uptake, the strains fell into three classes according to the amount of hydrogen peroxide (H2O2) released. Strains BHT, FA-1, and OMZ-176 released up to 90% of O2 taken up as H2O2, which accumulated in the medium to concentrations as high as 2 mM. The high levels of H2O2 accumulation were correlated with low ability to reduce exogenous H2O2 to water. Strains Ingbritt and B-13 released about half as much H2O2, but H2O2 in the medium did not exceed 0.05 to 0.1 mM. Strains HS-6, AHT, GS-5, OMZ-175, LM-7, and 6515-15 released less than 10% of O2 taken up as H2O2, and H2O2 did not accumulate. Within this class, strains HS-6 and AHT released about 6% of O2 taken up as superoxide (O2-). Release of O2 metabolites was correlated with enzyme activities in cell-free extracts. Extracts from all strains catalyzed NADH-dependent O2 uptake. Extracts from H2O2-accumulating strains produced H2O2 when incubated with NADH and O2 and had low ability to catalyze NADH-dependent reduction of H2O2. Extracts from HS-6 and AHT had low superoxide dismutase activity, which may account for O2- release and the O2-sensitive growth of these strains.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anders R. F., Hogg D. M., Jago G. R. Formation of hydrogen peroxide by group N streptococci and its effect on their growth and metabolism. Appl Microbiol. 1970 Apr;19(4):608–612. doi: 10.1128/am.19.4.608-612.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Archibald F. S., Fridovich I. The scavenging of superoxide radical by manganous complexes: in vitro. Arch Biochem Biophys. 1982 Apr 1;214(2):452–463. doi: 10.1016/0003-9861(82)90049-2. [DOI] [PubMed] [Google Scholar]
- Babior B. M. Oxygen-dependent microbial killing by phagocytes (second of two parts). N Engl J Med. 1978 Mar 30;298(13):721–725. doi: 10.1056/NEJM197803302981305. [DOI] [PubMed] [Google Scholar]
- Badwey J. A., Karnovsky M. L. Active oxygen species and the functions of phagocytic leukocytes. Annu Rev Biochem. 1980;49:695–726. doi: 10.1146/annurev.bi.49.070180.003403. [DOI] [PubMed] [Google Scholar]
- Boveris A., Martino E., Stoppani A. O. Evaluation of the horseradish peroxidase-scopoletin method for the measurement of hydrogen peroxide formation in biological systems. Anal Biochem. 1977 May 15;80(1):145–158. doi: 10.1016/0003-2697(77)90634-0. [DOI] [PubMed] [Google Scholar]
- Britton L., Malinowski D. P., Fridovich I. Superoxide dismutase and oxygen metabolism in Streptococcus faecalis and comparisons with other organisms. J Bacteriol. 1978 Apr;134(1):229–236. doi: 10.1128/jb.134.1.229-236.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole J. A. A biochemical approach to the control of dental caries. Biochem Soc Trans. 1977;5(4):1232–1239. doi: 10.1042/bst0051232. [DOI] [PubMed] [Google Scholar]
- Coykendall A. L. Four types of Streptococcus mutans based on their genetic, antigenic and biochemical characteristics. J Gen Microbiol. 1974 Aug;83(2):327–338. doi: 10.1099/00221287-83-2-327. [DOI] [PubMed] [Google Scholar]
- DOLIN M. I. Oxidation of reduced diphosphopyridine nucleotide by Clostridium perfringens. I. Relation of peroxide to the overall reaction. J Bacteriol. 1959 Apr;77(4):383–392. doi: 10.1128/jb.77.4.383-392.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiGuiseppi J., Fridovich I. Oxygen toxicity in Streptococcus sanguis. The relative importance of superoxide and hydroxyl radicals. J Biol Chem. 1982 Apr 25;257(8):4046–4051. [PubMed] [Google Scholar]
- Donoghue H. D., Tyler J. E. Antagonisms amongst streptococci isolated from the human oral cavity. Arch Oral Biol. 1975 May-Jun;20(5-6):381–387. doi: 10.1016/0003-9969(75)90031-x. [DOI] [PubMed] [Google Scholar]
- Fridovich I. Quantitative aspects of the production of superoxide anion radical by milk xanthine oxidase. J Biol Chem. 1970 Aug 25;245(16):4053–4057. [PubMed] [Google Scholar]
- Fridovich I. Superoxide dismutases. Annu Rev Biochem. 1975;44:147–159. doi: 10.1146/annurev.bi.44.070175.001051. [DOI] [PubMed] [Google Scholar]
- Hamada S., Slade H. D. Biology, immunology, and cariogenicity of Streptococcus mutans. Microbiol Rev. 1980 Jun;44(2):331–384. doi: 10.1128/mr.44.2.331-384.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamon C. B., Klebanoff S. J. A peroxidase-mediated, streptococcus mitis-dependent antimicrobial system in saliva. J Exp Med. 1973 Feb 1;137(2):438–450. doi: 10.1084/jem.137.2.438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmberg K., Hallander H. O. Production of bactericidal concentrations of hydrogen peroxide by Streptococcus sanguis. Arch Oral Biol. 1973 Mar;18(3):423–434. doi: 10.1016/0003-9969(73)90167-2. [DOI] [PubMed] [Google Scholar]
- Kaplan E. L., Laxdal T., Quie P. G. Studies of polymorphonuclear leukocytes from patients with chronic granulomatous disease of childhood: bactericidal capacity for streptococci. Pediatrics. 1968 Mar;41(3):591–599. [PubMed] [Google Scholar]
- Kral T. A., Daneo-Moore L. Biochemical differentiation of certain oral streptococci. J Dent Res. 1981 Sep;60(9):1713–1718. doi: 10.1177/00220345810600091301. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MASSEY V. The microestimation of succinate and the extinction coefficient of cytochrome c. Biochim Biophys Acta. 1959 Jul;34:255–256. doi: 10.1016/0006-3002(59)90259-8. [DOI] [PubMed] [Google Scholar]
- Mandell G. L., Hook E. W. Leukocyte bactericidal activity in chronic granulomatous disease: correlation of bacterial hydrogen peroxide production and susceptibility to intracellular killing. J Bacteriol. 1969 Oct;100(1):531–532. doi: 10.1128/jb.100.1.531-532.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCord J. M., Fridovich I. Superoxide dismutase. An enzymic function for erythrocuprein (hemocuprein). J Biol Chem. 1969 Nov 25;244(22):6049–6055. [PubMed] [Google Scholar]
- Ritchey T. W., Seely H. W., Jr Distribution of cytochrome-like respiration in streptococci. J Gen Microbiol. 1976 Apr;93(2):195–203. doi: 10.1099/00221287-93-2-195. [DOI] [PubMed] [Google Scholar]
- Terleckyj B., Willett N. P., Shockman G. D. Growth of several cariogenic strains of oral streptococci in a chemically defined medium. Infect Immun. 1975 Apr;11(4):649–655. doi: 10.1128/iai.11.4.649-655.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas E. L., Bates K. P., Jefferson M. M. Peroxidase antimicrobial system of human saliva: requirements for accumulation of hypothiocyanite. J Dent Res. 1981 Apr;60(4):785–796. doi: 10.1177/00220345810600040401. [DOI] [PubMed] [Google Scholar]
- Thomas E. L., Pera K. A., Smith K. W., Chwang A. K. Inhibition of Streptococcus mutans by the lactoperoxidase antimicrobial system. Infect Immun. 1983 Feb;39(2):767–778. doi: 10.1128/iai.39.2.767-778.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vance P. G., Keele B. B., Jr, Rajagopalan K. V. Superoxide dismutase from Streptococcus mutans. Isolation and characterization of two forms of the enzyme. J Biol Chem. 1972 Aug 10;247(15):4782–4786. [PubMed] [Google Scholar]


