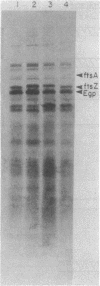
Abstract
Treatments that damage DNA in Escherichia coli result in the inhibition of cell division. This inhibition is controlled by the lexA-recA regulatory circuit and can be specifically uncoupled by the mutations sulA (sfiA) and sulB (sfiB), which map at 21 and 2 min, respectively. Presently it is thought that sulA codes for an inducible inhibitor of cell division, the expression of which is controlled directly by the lexA repressor. In this report, it is shown that sulB is an allele of ftsZ, an essential cell division gene. A sulB mutation leads to an altered ftsZ gene product which is slightly thermosensitive and has an altered mobility on polyacrylamide gels. It is suggested that the altered ftsZ gene product is resistant to the sulA inhibitor, thus permitting cell division after induction of the SOS response. It is also shown that an increase in the gene dosage of ftsZ delays the onset of filamentation after SOS induction.
Full text
PDF


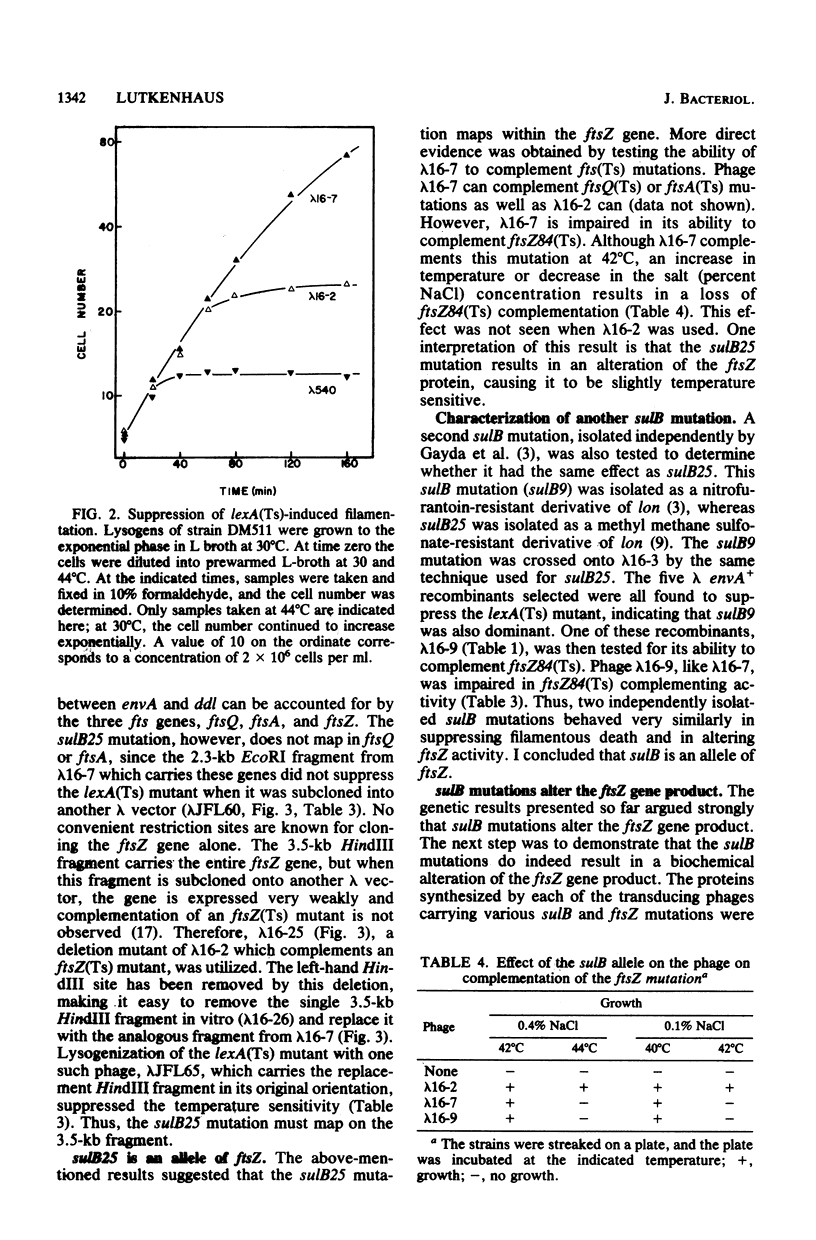




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Begg K. J., Hatfull G. F., Donachie W. D. Identification of new genes in a cell envelope-cell division gene cluster of Escherichia coli: cell division gene ftsQ. J Bacteriol. 1980 Oct;144(1):435–437. doi: 10.1128/jb.144.1.435-437.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burdett I. D., Murray R. G. Septum formation in Escherichia coli: characterization of septal structure and the effects of antibiotics on cell division. J Bacteriol. 1974 Jul;119(1):303–324. doi: 10.1128/jb.119.1.303-324.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gayda R. C., Yamamoto L. T., Markovitz A. Second-site mutations in capR (lon) strains of Escherichia coli K-12 that prevent radiation sensitivity and allow bacteriophage lambda to lysogenize. J Bacteriol. 1976 Sep;127(3):1208–1216. doi: 10.1128/jb.127.3.1208-1216.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George J., Castellazzi M., Buttin G. Prophage induction and cell division in E. coli. III. Mutations sfiA and sfiB restore division in tif and lon strains and permit the expression of mutator properties of tif. Mol Gen Genet. 1975 Oct 22;140(4):309–332. [PubMed] [Google Scholar]
- Gottesman S., Halpern E., Trisler P. Role of sulA and sulB in filamentation by lon mutants of Escherichia coli K-12. J Bacteriol. 1981 Oct;148(1):265–273. doi: 10.1128/jb.148.1.265-273.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOWARD-FLANDERS P., SIMSON E., THERIOT L. A LOCUS THAT CONTROLS FILAMENT FORMATION AND SENSITIVITY TO RADIATION IN ESCHERICHIA COLI K-12. Genetics. 1964 Feb;49:237–246. doi: 10.1093/genetics/49.2.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huisman O., D'Ari R. An inducible DNA replication-cell division coupling mechanism in E. coli. Nature. 1981 Apr 30;290(5809):797–799. doi: 10.1038/290797a0. [DOI] [PubMed] [Google Scholar]
- Huisman O., D'Ari R., George J. Further characterization of sfiA and sfiB mutations in Escherichia coli. J Bacteriol. 1980 Oct;144(1):185–191. doi: 10.1128/jb.144.1.185-191.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson B. F. Fine structure mapping and properties of mutations suppressing the lon mutation in Escherichia coli K-12 and B strains. Genet Res. 1977 Dec;30(3):273–286. doi: 10.1017/s0016672300017687. [DOI] [PubMed] [Google Scholar]
- Jones N. C., Donachie W. D. Chromosome replication, transcription and control of cell division in Escherichia coli. Nat New Biol. 1973 May 23;243(125):100–103. [PubMed] [Google Scholar]
- Kirby E. P., Ruff W. L., Goldthwait D. A. Cell division and prophage induction in Escherichia coli: effects of pantoyl lactone and various furan derivatives. J Bacteriol. 1972 Aug;111(2):447–453. doi: 10.1128/jb.111.2.447-453.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leighton P. M., Donachie W. D. Deoxyribonucleic acid synthesis and cell division in a lon- mutant of Escherichia coli. J Bacteriol. 1970 Jun;102(3):810–814. doi: 10.1128/jb.102.3.810-814.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little J. W., Edmiston S. H., Pacelli L. Z., Mount D. W. Cleavage of the Escherichia coli lexA protein by the recA protease. Proc Natl Acad Sci U S A. 1980 Jun;77(6):3225–3229. doi: 10.1073/pnas.77.6.3225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little J. W., Harper J. E. Identification of the lexA gene product of Escherichia coli K-12. Proc Natl Acad Sci U S A. 1979 Dec;76(12):6147–6151. doi: 10.1073/pnas.76.12.6147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little J. W., Mount D. W. The SOS regulatory system of Escherichia coli. Cell. 1982 May;29(1):11–22. doi: 10.1016/0092-8674(82)90085-x. [DOI] [PubMed] [Google Scholar]
- Lutkenhaus J. F., Donachie W. D. Identification of the ftsA gene product. J Bacteriol. 1979 Mar;137(3):1088–1094. doi: 10.1128/jb.137.3.1088-1094.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutkenhaus J. F., Wu H. C. Determination of transcriptional units and gene products from the ftsA region of Escherichia coli. J Bacteriol. 1980 Sep;143(3):1281–1288. doi: 10.1128/jb.143.3.1281-1288.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mound D. W., Walker A. C., Kosel C. Suppression of lex mutations affecting deoxyribonucleic acid repair in Escherichia coli K-12 by closely linked thermosensitive mutations. J Bacteriol. 1973 Nov;116(2):950–956. doi: 10.1128/jb.116.2.950-956.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phizicky E. M., Roberts J. W. Induction of SOS functions: regulation of proteolytic activity of E. coli RecA protein by interaction with DNA and nucleoside triphosphate. Cell. 1981 Jul;25(1):259–267. doi: 10.1016/0092-8674(81)90251-8. [DOI] [PubMed] [Google Scholar]
- Salmond G. P., Lutkenhaus J. F., Donachie W. D. Identification of new genes in a cell envelope-cell division gene cluster of Escherichia coli: cell envelope gene murG. J Bacteriol. 1980 Oct;144(1):438–440. doi: 10.1128/jb.144.1.438-440.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker J. R., Kovarik A., Allen J. S., Gustafson R. A. Regulation of bacterial cell division: temperature-sensitive mutants of Escherichia coli that are defective in septum formation. J Bacteriol. 1975 Aug;123(2):693–703. doi: 10.1128/jb.123.2.693-703.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wijsman H. J., Koopman C. R. The relation of the genes envA and ftsA in Escherichia coli. Mol Gen Genet. 1976 Aug 10;147(1):99–102. doi: 10.1007/BF00337942. [DOI] [PubMed] [Google Scholar]