Abstract
Rationale: High-dose ibuprofen in a 4-year controlled trial slowed FEV1 decline in young subjects with cystic fibrosis, but the effectiveness of ibuprofen has not been assessed in a large group of patients treated clinically with this therapy.
Objectives: To assess the effect of ibuprofen therapy on FEV1 decline in children and adolescents with cystic fibrosis, using observational data from the Cystic Fibrosis Foundation Patient Registry.
Methods: The rate of decline in FEV1 percent predicted over 2–7 years among patients age 6–17 years with FEV1 > 60% predicted, and who were treated with ibuprofen (1,365), was compared with patients of similar age and disease severity who were not treated with this therapy (8,960). Multilevel repeated-measures mixed-regression models were used to estimate rates of decline, adjusting for characteristics and therapies that influenced FEV1 decline. Adverse effects were compared among those treated versus not treated with ibuprofen.
Measurements and Main Results: FEV1 declined less rapidly among patients treated with ibuprofen (difference, 0.60% predicted per year; 95% confidence interval, 0.31 to 0.89; P < 0.0001); a 29% reduction in slope based on an average decline of 2.08% predicted per year for patients not treated. Those treated with ibuprofen were more likely to have an episode of gastrointestinal bleeding requiring hospitalization, but the occurrence was rare in both groups (annual incidence, 0.37 vs. 0.14%; relative risk, 2.72; P < 0.001).
Conclusions: Slower rates of FEV1 decline are seen in children and adolescents with cystic fibrosis who are treated with ibuprofen. The apparent benefits of ibuprofen therapy outweigh the small risk of gastrointestinal bleeding.
Keywords: cystic fibrosis, ibuprofen, antiinflammatory therapy, pulmonary function, epidemiology
AT A GLANCE COMMENTARY
Scientific Knowledge on the Subject
Inflammation contributes to lung damage, shortening survival of patients with cystic fibrosis (CF). FEV1 decline predicts survival in CF. Ibuprofen taken over 4 years was shown in a controlled clinical trial to slow the rate of FEV1 decline in children with CF.
What This Study Adds to the Field
Real-world clinical use of ibuprofen is associated with a slower rate of FEV1 decline in children with CF. These results should lead to an increase in the use of ibuprofen among patients with CF.
High-dose ibuprofen was shown in a randomized, double-blind, placebo-controlled trial to reduce the rate of decline of pulmonary function in patients with cystic fibrosis (CF) with good to excellent pulmonary function (1). During this trial, considerable attention was paid to adherence, there was emphasis on assiduous follow-up, dosage was regularly adjusted according to pharmacokinetic criteria, and pulmonary function testing was done under rigorously controlled conditions. In the clinical setting, however, control of these factors is not nearly as rigorous. Ibuprofen may be ineffective and possibly harmful in subtherapeutic doses (2), does not have immediate therapeutic benefit that is perceptible to the patient, and has some risk of adverse effects. Thus, looser monitoring of patients might well give less favorable results. In the original clinical trial, results were better when restricted to subjects with more than 70% treatment adherence than they were for the intent-to-treat groups, suggesting that spotty compliance or going on and off the drug may reduce its effectiveness. Moreover, a number of new drugs have been introduced for the treatment of CF, which were not in use during the clinical trial, and it is conceivable that these drugs could confound the effectiveness of ibuprofen.
To examine whether high-dose ibuprofen is as effective when it is used in the clinical setting as it was under the highly controlled conditions of the original trial, we turned to the Cystic Fibrosis Foundation (Bethesda, MD) Patient Registry (CFF Registry), which records information on all consenting patients with CF monitored at U.S. CF centers (3). We identified groups of patients in the proper age and severity range who were or were not treated with ibuprofen for antiinflammatory purposes, and determined their rate of decline of pulmonary function. We identified the factors that influenced the rate of decline of pulmonary function in this group of patients, and controlled for them in our analysis. Some of the results of this study have been previously reported in the form of an abstract (4).
METHODS
Data analyzed were obtained from the CFF Registry. We included data from 1996 (the first year patients would have received ibuprofen for an entire year) through 2002, the last year for which data were available. The analysis included patients who had at least two consecutive years with ibuprofen use or nonuse, with FEV1 measured at the initial (baseline) year and at least one subsequent year. For patients treated with ibuprofen, the baseline year was defined as the first year they received ibuprofen. For patients not treated or who remained continuously on ibuprofen, follow-up extended to the last year when FEV1 was measured. For those who discontinued treatment, follow-up extended to the year before the year ibuprofen was discontinued. Thus, each patient's data spanned a period when he/she was either always treated or not treated with ibuprofen. We further restricted the analysis to include only patients age 6–17 years with FEV1 > 60% predicted at baseline, because the original clinical trial suggested a benefit of ibuprofen in this subgroup (1). Patients ever infected with Burkholderia cepacia, and any data obtained after organ transplantation, were excluded.
Variable Definitions
FEV1 percent predicted (5) was provided as the average of quarterly measurements during the year. Values greater than 140 were set to missing. Pseudomonas aeruginosa infection status was coded as (1) negative at baseline and follow-up, (2) negative at baseline but later positive, or (3) positive at baseline. Weight-for-age percentile based on Centers for Disease Control and Prevention (Atlanta, GA) norms was used as a measure of baseline nutritional status. The number of CF-related hospitalizations in the year before baseline was categorized as 0, 1, 2, or 3 or more. Pancreatic enzyme use was coded as yes if the patient ever reported receiving enzymes in any year, and as no otherwise. Baseline insurance status was dichotomized: no insurance or state/federal insurance only versus other insurance. Median family income based on residence postal code at baseline was obtained from the 2000 census.
Statistical Analysis of FEV1 Data
A longitudinal multilevel mixed-effects regression model was used to analyze changes from baseline in FEV1 percent predicted. Preliminary analyses found that the pattern of change in FEV1 percent predicted from baseline was not linear, and varied according to baseline age and FEV1 percent predicted. Thus, changes from baseline in FEV1 percent predicted were analyzed as a quadratic function of time since baseline, specifying a different quadratic curve for each of the 12 strata defined by baseline age (<8, 8–12, and 13–17 yr) and FEV1 percent predicted (60–79, 80–89, 90–99, and ⩾100%). In addition, the multilevel model included random effects for the intercepts, slopes, and quadratic coefficients of individuals (level 1), and for the CF centers and affiliate centers where individuals received their care (level 2). Effects of ibuprofen and other covariates on slope and quadratic trend in FEV1 percent predicted were examined by including interactions between the predictors and time or (time)2, respectively, where time = years since baseline. Only baseline FEV1 percent predicted and age were found to be related to quadratic trends in FEV1 percent predicted; other covariates including ibuprofen use (yes/no) were thus modeled only in terms of their effects on linear rate of decline in FEV1. Including clinical center as a random effect controlled for possible confounding effects of clinical center when examining the relationship between ibuprofen use and FEV1 decline (as would occur if centers with patients with more/less severe disease were more/less likely to prescribe ibuprofen, after controlling for other factors in the model). Cumulative and acute effects of concurrent treatment with inhaled tobramycin and dornase alfa were examined with time-varying covariates representing yearly use or nonuse, and cumulative years of use since baseline, respectively. See the online supplement for further details of the longitudinal statistical model.
Adverse Events
The annual incidence of gastrointestinal ulcers, gastrointestinal bleeding requiring hospitalization, and renal failure requiring dialysis was determined for patients treated and not treated with ibuprofen. This analysis pooled multiple years using a binomial generalized estimating equations model with log link function.
Analyses were performed with SAS version 9.1 (SAS Institute, Cary, NC). The study was approved by the Institutional Review Board of University Hospitals of Cleveland.
RESULTS
During 1996–2002, 17,175 patients at 172 CF centers (including affiliates) had two or more annual FEV1 measurements spanning a period during which ibuprofen use or nonuse was continuously recorded, after excluding transplantation patients and those infected with B. cepacia. Of these, 1,936 (11%) were treated with ibuprofen, of whom 511 remained on therapy and 1,425 discontinued for at least 1 year after initiating therapy. Restricting the cohort to those age 6–17 (<18) years with baseline FEV1 > 60% predicted resulted in a sample of 10,325 of whom 411 (4.0%) initiated and remained on therapy, 954 (9.2%) initiated but later discontinued therapy for at least 1 year, and 8,960 (86.8%) were never treated with ibuprofen. Ninety-two (53%) centers treated patients with ibuprofen.
Baseline characteristics of patients who were treated versus not treated with ibuprofen are summarized in Table 1. The groups did not differ with respect to mean FEV1 percent predicted or mean weight-for-age percentile. However, those treated with ibuprofen were more likely to be older, female, infected with P. aeruginosa, had more hospitalizations in the year before baseline, had higher mean income, were less likely to have no insurance or state/federal insurance only, and more likely to be treated with inhaled tobramycin and dornase alfa. More than 98% of patients in both groups were taking or had been treated with pancreatic enzymes.
TABLE 1.
BASELINE CHARACTERISTICS OF PATIENTS TREATED VERSUS NOT TREATED WITH IBUPROFEN
| Treated | Not Treated | P Value* | |
|---|---|---|---|
| Baseline age, mean ± SD (n) | 10.2 ± 3.2 (1,365) | 9.7 ± 3.6 (8,960) | <0.0001 |
| 6–12 yr, % | 78.6 | 77.8 | 0.52 |
| 13–18 yr, % | 21.4 | 22.2 | |
| Male, % | 49.3% | 53.3% | 0.006 |
| Baseline FEV1 percent predicted, mean ± SD (n) | 92.1 ± 15.7 (1,365) | 92.5 ± 16.7 (8,960) | 0.73 |
| 60–79%pred, % | 24.8 | 24.9 | 0.48 |
| 80–89%pred, % | 19.7 | 20.4 | |
| 90–99%pred, % | 24.2 | 22.3 | |
| ⩾100%pred, % | 31.4 | 31.4 | |
| Weight-for-age percentile, mean ± SD (n) | 36.5 ± 26.4 (1,365) | 36.2 ± 26.8 (8,956) | 0.47 |
| Pseudomonas Aeruginosa infection | |||
| Never infected, % | 13.0 | 27.1 | <0.0001 |
| Infected after baseline, % | 22.5 | 24.5 | |
| Always infected, % | 62.3 | 44.4 | |
| Unknown baseline, later positive, % | 2.1 | 4.0 | |
| Hospitalizations in prior year, mean ± SD (n) | 0.36 ± 0.82 (1,301) | 0.30 ± 0.74 (7,610) | 0.010 |
| Median annual income ($), mean ± SD (n) | 48,527 ± 17,909 (1,326) | 46,063 ± 16,633 (8,633) | <0.0001 |
| No or state/federal insurance only, % | 20.2 | 30.3 | <0.0001 |
| Inhaled tobramycin use | |||
| At baseline, % | 16.5 | 10.1 | <0.0001 |
| Ever during follow-up, % | 76.7 | 61.3 | <0.0001 |
| Dornase alfa use | |||
| At baseline, % | 50.9 | 39.4 | <0.0001 |
| Ever during follow-up, % | 79.2 | 69.9 | <0.0001 |
P values from Wilcoxon rank sum test or chi-square test.
Baseline characteristics of patients who discontinued ibuprofen versus those who remained on this therapy for the duration of follow-up are summarized in Table 2. Patients who were older, had lower mean FEV1 percent predicted and lower mean weight-for-age percentile, or had more CF hospitalizations in the year before baseline were more likely to discontinue ibuprofen. Those who discontinued were also more likely to be treated with dornase alfa at baseline and during follow-up, but less likely to be treated with inhaled tobramycin at baseline.
TABLE 2.
BASELINE CHARACTERISTICS OF PATIENTS WHO CONTINUED VERSUS DISCONTINUED IBUPROFEN THERAPY
| Continued | Discontinued | P Value* | |
|---|---|---|---|
| Baseline age (yr), mean ± SD (n) | 9.2 ± 2.9 (411) | 10.6 ± 3.2 (954) | <0.0001 |
| 6–12 yr, % | 86.6 | 75.2 | <0.0001 |
| 13–18 yr, % | 13.4 | 24.8 | |
| Male, % | 52.6 | 47.9 | 0.12 |
| Baseline FEV1 percent predicted, mean ± SD (n) | 93.7 ± 16.1 (411) | 91.3 ± 15.5 (954) | 0.0147 |
| 60–79%pred, % | 22.4 | 25.8 | 0.17 |
| 80–89%pred, % | 17.5 | 20.6 | |
| 90–99%pred, % | 26.5 | 23.2 | |
| ⩾100%pred, % | 33.6 | 30.4 | |
| Weight-for-age percentile, mean ± SD (n) | 38.7 ± 26.5 (411) | 35.5 ± 26.2 (954) | 0.037 |
| Pseudomonas aeruginosa infection | |||
| Never infected, % | 15.6 | 11.9 | 0.058 |
| Infected after baseline, % | 23.6 | 22.0 | |
| Always infected, % | 59.9 | 63.4 | |
| Unknown baseline, later positive, % | 1.0 | 2.6 | |
| Hospitalizations in prior year, mean ± SD (n) | 0.26 ± 0.78 (388) | 0.40 ± 0.83 (913) | 0.0004 |
| Median annual income ($), mean ± SD (n) | 47,538 ± 17,694 (402) | 48,957 ± 17,995 (924) | 0.13 |
| No or state/federal insurance only, % | 19.0 | 20.7 | 0.48 |
| Inhaled tobramycin use | |||
| At baseline, % | 21.2 | 14.5 | 0.0022 |
| Ever during follow-up, % | 74.7 | 77.6 | 0.25 |
| Dornase alfa use | |||
| At baseline, % | 45.5 | 53.2 | 0.0086 |
| Ever during follow-up, % | 75.2 | 80.9 | 0.017 |
P values from Wilcoxon rank sum test or chi-square test.
Longitudinal Decline in FEV1
The final longitudinal statistical model for change in FEV1 percent predicted included linear and quadratic terms to the 12 strata of baseline age and FEV1 percent predicted, as well as terms representing the effect of the following covariates on linear rate of decline in FEV1 percent predicted: sex, weight-for-age percentile, number of hospitalizations in the year before baseline, P. aeruginosa status, baseline insurance status, and whether they were treated with ibuprofen or not treated. All of these terms were significant predictors of yearly rate of decline in FEV1 percent predicted in the multivariable model. Pancreatic enzyme use and median income were not significant and thus were not included in the model. Random effects for clinical center and patient were also statistically significant; all patient-specific and center-specific variance components were significantly greater than zero (P < 0.0001), indicating significant between-center and between-patient variability in linear and quadratic trends in FEV1 percent predicted. Estimated variance components (SEs) for linear and quadratic variance components were 3.77 (0.85) and 0.07 (0.02), respectively, for between-center effects, and 31.3 (2.1) and 0.52 (0.04) for between-patient effects within center. Thus, approximately 11% [3.77/(3.77 + 31.3)] and 12% [0.07/(0.07 + 0.52)] of the total between-patient variability in linear and quadratic trends of FEV1 percent predicted was due to center-to-center variability. Because of exclusion of patients missing one or more of the covariates, the final multivariable model included 8,185 patients and 35,500 follow-up observations.
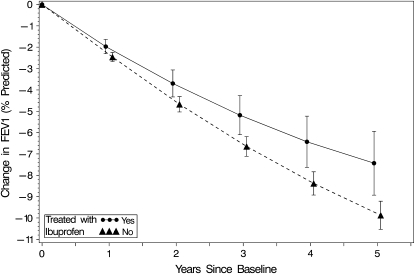
Results, summarized in Table 3, show that FEV1 decline was more rapid among females, and those infected with P. aeruginosa, those with lower weight-for-age percentile, those with more prior CF hospitalizations, and those with no or only state/federal insurance at baseline. In addition, quadratic patterns of change in FEV1 existed, and differed according to baseline age and FEV1. Both age × FEV1 × time, and age × FEV1 × (time)2, were significant (P < 0.0001). Random effects for CF center, as well as for individual patients within center, were statistically significant (P < 0.001) and were included in the model. After adjusting for all factors listed in Table 3 as well as for nonlinear quadratic patterns of change in FEV1 percent predicted stratified by baseline age and level of FEV1 percent predicted, FEV1 declined less rapidly among patients who were treated with ibuprofen (difference, 0.48% predicted per year; 95% confidence interval [CI], 0.19 to 0.78; P = 0.0013). Figure 1 presents estimates of mean change from baseline in FEV1 percent predicted, representing the average quadratic curves for patients treated and not treated with ibuprofen, adjusting for baseline covariates affecting FEV1 decline (model in Table 3). Average 5-year changes in FEV1 (±SE) were −7.43 ± 0.76 and −9.87 ± 0.36% predicted for those treated versus not treated with ibuprofen, respectively.
TABLE 3.
LONGITUDINAL MULTIVARIABLE STATISTICAL MODEL EXAMINING FACTORS RELATED TO YEARLY RATE OF DECLINE IN FEV1 PERCENT PREDICTED
| Variable | Estimate* | Standard Error | P Value |
|---|---|---|---|
| Ibuprofen use | |||
| Treated | 0.4818 | 0.1496 | 0.0013 |
| Not treated | 0 | ||
| Sex | |||
| Male | 0.3455 | 0.0680 | <0.0001 |
| Female | 0 | ||
| Pseudomonas aeruginosa infection | |||
| Never infected | 1.2239 | 0.0981 | <0.0001 |
| Infected after baseline | 0.6190 | 0.0818 | |
| Infected at baseline | 0 | ||
| Baseline weight-for-age percentile | 0.0160 | 0.0013 | <0.0001 |
| Baseline insurance | |||
| None or state/federal only | −0.2601 | 0.0781 | 0.0009 |
| All other | 0 | ||
| No. of previous CF hospitalizations | |||
| None | 1.2466 | 0.2397 | <0.0001 |
| 1 | 0.8693 | 0.2506 | |
| 2 | 0.6545 | 0.2878 | |
| 3 or more | 0 |
Model also includes interactions of age × FEV1 stratum with time and (time)2 (see Methods).
For categorical variables, the estimate is the change in rate of decline in FEV1 percent predicted per year between the indicated group and the reference group (labeled by estimate = 0). For continuous covariates it is the change in rate of decline in FEV1 percent predicted per year when the covariate increases by 1 unit.
Figure 1.
Estimated average change from baseline in FEV1 percent predicted over a 5-year period of observation for patients treated (1,365) and not treated (8,960) with high-dose ibuprofen. Patients were age 6–17 years with FEV1 exceeding 60% predicted at baseline. The actual observation period for each patient ranged from 2 to 7 years, and occurred from 1996 to 2002. Data points are the adjusted mean changes in FEV1 percent predicted (with 95% confidence interval) at each year after baseline, determined from the longitudinal model (Table 3), adjusting for baseline FEV1 percent predicted, age, and other baseline covariates (not including use of inhaled tobramycin or dornase alfa).
We also estimated the average rate of FEV1 decline among those not treated with ibuprofen in the registry by fitting a mixed linear model without covariates, and found it to be 2.08% predicted per year. Thus, on average, a reduction in slope of 0.48% predicted per year due to treatment with ibuprofen would correspond to a rate of decline of 1.60% predicted per year. This represents a 23% reduction in slope. Interactions between ibuprofen group (treated vs. not treated) and each of the covariates in Table 3, as well as with baseline age and FEV1 groups, were tested and none was significant (all P values > 0.28). When included in the model, a quadratic effect of ibuprofen was also not significant (P = 0.55).
To adjust for concurrent therapy with inhaled tobramycin and/or dornase alfa, we fit the same model as in Table 3, but with terms added to represent current (i.e., acute) and cumulative effects of both inhaled tobramycin and dornase alfa. Neither therapy showed a significant acute effect (i.e., a transient increase or decrease in FEV1 percent predicted while taking the drug, that disappears when the drug is discontinued). However, patients who were receiving these therapies were found to have more rapid rates of decline in FEV1 compared with those not receiving them (yearly effects of −0.93% predicted per year for inhaled tobramycin, and −0.43% predicted per year for dornase alfa; P < 0.0001 for both). These results suggest that there may be a selection bias toward treating sicker patients with these medications, as has been shown for dornase alfa (6). In any case, adjusting for inhaled tobramycin and dornase alfa in this way strengthened the effect of ibuprofen treatment, with those treated having slopes less negative by 0.60% predicted per year (95% CI, 0.31 to 0.89; P = 0.0001). This represents a 29% reduction in slope for patients treated with ibuprofen, based on an average decline of 2.08% predicted per year for patients not treated with ibuprofen.
We also fit a model identical to the one in Table 3, with the exception that the group receiving ibuprofen was split into those who remained on therapy (always on) and those who discontinued therapy. In this model, compared with those not treated with ibuprofen, those always on ibuprofen had a less negative decline in FEV1 percent predicted (difference, 0.63% predicted per year; 95% CI, 0.27 to 1.00% predicted per year; P = 0.0007), representing an average 30% reduction in slope. Although the rate of decline while taking ibuprofen, among those who subsequently discontinued therapy, was less negative than the slope of those not treated with ibuprofen, the difference was not statistically significant (difference, 0.27% predicted per year; 95% CI, −0.16 to 0.71; P = 0.21).
The findings that, among those patients who received ibuprofen, (1) those who discontinued therapy tended to be sicker and (2) those who continued to take ibuprofen showed less negative rates of decline while taking ibuprofen compared with those who discontinued therapy, both raise the possibility that patients who discontinued ibuprofen might be a form of informative dropout (7). This type of informative dropout could bias the results toward a beneficial effect of ibuprofen because the sicker patients who discontinue ibuprofen would have shorter follow-up times and would be given disproportionately less weight in the analysis compared with healthy patients who continue to take ibuprofen. To investigate this possibility, we fit a “pattern mixture” model (8), which grouped patients according to ibuprofen use pattern (always on, discontinued, or never on ibuprofen) and according to length of follow-up within each ibuprofen group. Mean slopes were estimated for each pattern, and then these means were weighted by the proportions in the groups to obtain estimates of the mean slopes for those on versus not on ibuprofen. These estimates are less susceptible to bias when informative dropout exists (8). The estimated effect of ibuprofen obtained from this approach was somewhat larger in magnitude compared with the estimate obtained from the analysis shown in Table 3, although the difference was not statistically significant (difference in slopes, 0.57% predicted per year; 95% CI, −0.08 to 0.84; P = 0.10). The similarity of the two estimates provides some reassurance that the estimated effect of ibuprofen reported in Table 3 is not biased because of selective dropout (discontinuation of ibuprofen).
Adverse Events
The annual incidence of adverse events among patients treated versus not treated with ibuprofen in a given year is summarized in Table 4. Although the incidence was low for all three events, a statistically significant difference between the two groups was observed for gastrointestinal bleeding requiring hospitalization. This adverse event occurred more frequently in patients treated with ibuprofen (annual incidence, 0.37%) versus those not treated (0.14%), with a relative risk of 2.72 for the occurrence of this adverse event among those treated with ibuprofen (P < 0.001). The differences in the incidence of gastrointestinal ulcers and renal failure requiring dialysis among patients who were treated versus not treated with ibuprofen were not statistically significant.
TABLE 4.
ANNUAL INCIDENCE OF ADVERSE EVENTS AMONG PATIENTS TREATED VERSUS NOT TREATED WITH IBUPROFEN
| Treated (4,340 PY) (%) | Not Treated (39,847 PY) (%) | Relative Risk (95% CI) | P Value | |
|---|---|---|---|---|
| Ulcers | 0.09 | 0.16 | 0.59 (0.21, 1.64) | 0.31 |
| GI bleeds | 0.37 | 0.14 | 2.72 (1.51, 4.89) | <0.001 |
| Renal failure | 0.05 | 0.02 | 2.30 (0.49, 10.8) | 0.29 |
Definition of abbreviations: CI = confidence interval; GI = gastrointestinal; PY = person-years.
DISCUSSION
The use of ibuprofen for antiinflammatory purposes by patients age 6–17 years with initial FEV1 > 60% predicted is associated with a significantly slower rate of decline of FEV1 percent predicted compared with patients not treated with ibuprofen. The reduction of 29% per year corresponds to preservation of greater than 1% predicted of FEV1 for every 2 years. This improvement is of the same order of magnitude as the improvement of rate of change of FEV1 associated with male sex or having private insurance as opposed to government-supported medical care. This improvement is less than reported under clinical trial conditions, but is still significant. Direct comparison of the 4-year controlled clinical trial data and our current data for absolute value of decline in FEV1 is difficult, however, because the clinical trial was conducted during 1988–1994, and the present data are from 1996 to 2002. Improved and earlier diagnosis, more aggressive use of therapies, as well as the introduction of new and effective therapeutic modalities during the interval are credited with improved outcomes for patients born later. In addition, results reported in the clinical trial are for an age group not precisely comparable to those studied here (ages 5–12 or >13 yr for the clinical trial, 6–17 yr for this study).
The use of CFF Registry data has several limitations. A single yes/no question is asked about the use of ibuprofen for antiinflammatory purposes each year. No information is available about the number of months the patient took the drug, or whether the dosage was actually adjusted according to pharmacokinetic parameters as recommended. No advice is given as to how the question should be answered if the patient discontinued the drug during that year, or initiated it at the end of the year. No information on adherence is available. Thus, included in the ibuprofen group may be patients who did not take the drug for the entire year, or who were nonadherent, or whose dose was not properly adjusted. CFF Registry data are not audited, so the correctness of the responses is not verified independently. Finally, we acknowledge that in analyzing observational registry data, there are inherent potential selection biases in terms of which patients receive or remain on ibuprofen, and we can never be certain that these have been completely controlled for in the analysis. However, many of the difficulties in the interpretation of the registry data would tend to disfavor ibuprofen (dose too low, or drug not taken for the entire year, or nonadherence), making it less likely that the limitations of the CFF Registry data produced a falsely good outcome for ibuprofen.
More detailed analysis of the data suggests that physicians may select patients in the appropriate age and pulmonary function status who are slightly less well for prescription of ibuprofen. Although there were no significant differences in baseline FEV1 between those treated versus not treated with ibuprofen, those treated were more likely to have been hospitalized in the prior year, significantly more likely to be infected with P. aeruginosa, and significantly more likely to be treated with either inhaled tobramycin or dornase alfa than those not treated with ibuprofen. That is, clinicians may intensify treatment (e.g., start ibuprofen) in response to worsening symptoms or declining status. Those who discontinued ibuprofen also appear to be older and sicker than those who remained on the drug. Their FEV1 was slightly lower, they were more likely to have been hospitalized, and to have poorer weight-for-age than those who remained on ibuprofen. These data imply that physicians may not be treating the healthiest, least affected patients (those who by many arguments might be most likely to benefit from ibuprofen) and may discontinue the drug if a patient's course appears progressive.
Another reason for discontinuing ibuprofen may be adverse effects. Adverse effects on two organs in particular, the gastrointestinal tract and kidney, have been cited by physicians as reasons to discontinue high-dose ibuprofen in patients with CF, or never to start it (9). Patients treated with ibuprofen had more than a twofold increased incidence of gastrointestinal hemorrhage compared with patients who were not treated. It is noteworthy that the incidence of gastrointestinal hemorrhage is elevated among patients with cystic fibrosis, even those who had never received ibuprofen. Patients with CF reportedly have more gastric and duodenal ulcers than their age-matched controls, and those with cirrhosis, which is seen in this age group, are more likely to have esophageal varices than other patients, which can bleed (10). Several case reports suggest an association of ibuprofen with gastrointestinal complications (11, 12), but causal relationships with ibuprofen treatment are difficult to prove, given that these complications can otherwise occur in CF. Although case reports identify renal failure occurring with high-dose ibuprofen in the setting of systemic aminoglycoside use and dehydration in children with CF undergoing treatment for pulmonary exacerbations (13, 14), renal failure was not borne out by analysis of the CFF Registry data.
The risk of adverse events associated with any drug is best assessed in a large number of patients over a long period of time. The number of patients in the 4-year controlled trial of ibuprofen was small, but they were carefully studied. The CFF Registry population is large, but the ability to track adverse effects related to ibuprofen is limited to those available for this analysis. Milder adverse events related to ibuprofen may include abdominal pain from undetected ulcers, small gastrointestinal bleeds not requiring hospitalization, or elevations in serum creatinine not progressing to renal failure. These would not be captured in the registry. Moreover, adverse events may not be predictable and may arise even after years of safe therapy. As previously noted, symptoms associated with ibuprofen occur in CF whether or not ibuprofen is consumed (e.g., abdominal pain, gastrointestinal bleeding). Patients taking ibuprofen may discontinue its use in response to such symptoms whether or not they are related to ibuprofen itself. Indeed, in earlier controlled trials, abdominal pain and the presence of occult blood in stools were just as common in placebo-treated subjects as they were in those treated with ibuprofen (1, 15). A report from a single center cited several gastrointestinal and other complications common to CF for discontinuing ibuprofen treatment in 36% of their 47 pediatric patients treated with ibuprofen, but did not report the incidence of these events among their untreated patients (16). Moreover, a single striking adverse event can change prescribing patterns in an entire center, so some of the high rate of discontinuation of ibuprofen therapy may result from physician preference. In addition, because subtherapeutic doses of ibuprofen may actually increase neutrophil delivery to a site of mucosal inflammation (2), physicians may choose to discontinue and not adjust dosage of ibuprofen in the face of minor adverse events.
One striking result of this study is the greater rate of decline in FEV1 that was observed for patients treated with inhaled tobramycin and/or dornase alfa. It is possible that patients who have a more rapidly progressive course are more likely to be treated with these drugs than those whose course is more indolent. Alternatively, once the initial improvement in FEV1 has occurred with these drugs, as reported in the trials of each of them, the rate of decline is either not altered or is actually accelerated. It is also possible that at least some patients met the FEV1 criteria for inclusion only because they were treated with inhaled tobramycin or dornase alfa, and were in fact sicker than the population included in the initial clinical trial. For these patients, the impact of ibuprofen is less certain. After adjusting for inhaled tobramycin and dornase alfa use, patients treated with ibuprofen had an even greater reduction in the rate of decline in FEV1 compared with those who were not treated.
This study represents the first use of the CFF Patient Registry to assess the long-term efficacy of a therapeutic intervention. Improvements in the CFF Registry, including encounter-based data reporting, should markedly improve its utility for such studies. Application of sophisticated statistical tools also allows us to capitalize on the strength of the large numbers in the registry without the handicap of its diversity. Assessing the real-world efficacy of a therapeutic intervention without the use of a large multicenter observational database is difficult, particularly if sufficient numbers of patients and/or years of observation are not included (17), and if adjustments for factors affecting the predefined outcome are not considered in the analysis. The single-center report of ibuprofen use in their population of children and adolescents with CF may not have demonstrated an association with change in the rate of FEV1 decline for these same reasons (16). For CF, such assessments are best left to analysis of large databases such as the CFF Patient Registry (3) or the Epidemiologic Study of Cystic Fibrosis (6, 18).
Our results suggest that even under real-world conditions, high-dose ibuprofen is of benefit to patients with cystic fibrosis who are age 6–17 years and have FEV1 > 60% predicted. The incidence of gastrointestinal bleeding is increased in those treated with ibuprofen, albeit low. Thus, data from the CFF Registry suggest that the benefit of high-dose ibuprofen therapy outweighs the small risks in children and adolescents with mild to moderate CF lung disease, and support the recommendation that ibuprofen therapy should be prescribed for such patients until an alternative antiinflammatory agent with a more favorable risk–benefit profile becomes available (19).
Supplementary Material
Acknowledgments
The authors thank Preston W. Campbell III, M.D., and Bruce C. Marshall, M.D., for providing the data from the Cystic Fibrosis Foundation Patient Registry and for critical review of the manuscript.
Supported by a Research Development Program Grant from the Cystic Fibrosis Foundation and by grant P30 DK27651 from the National Institutes of Health.
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1164/rccm.200702-181OC on September 13, 2007
Conflict of Interest Statement: M.W.K. served as a consultant to Genentech and received honoraria as co-chair of the scientific advisory board for the Epidemiologic Study of Cystic Fibrosis (sponsored by Genentech), and received honoraria for serving on advisory boards for Chiron and Novartis. M.D.S. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. W.X. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. P.B.D. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Konstan MW, Byard PJ, Hoppel CL, Davis PB. Effect of high-dose ibuprofen in patients with cystic fibrosis. N Engl J Med 1995;332:848–854. [DOI] [PubMed] [Google Scholar]
- 2.Konstan MW, Krenicky JE, Finney MR, Kirchner HL, Hilliard KA, Hilliard JB, Davis PB, Hoppel CL. Effect of ibuprofen on neutrophil migration in vivo in cystic fibrosis and healthy subjects. J Pharmacol Exp Ther 2003;306:1086–1091. [DOI] [PubMed] [Google Scholar]
- 3.Cystic Fibrosis Foundation. Cystic Fibrosis Foundation Patient Registry: 2002 annual data report. Bethesda, MD: Cystic Fibrosis Foundation; 2002.
- 4.Schluchter MD, Konstan MW, Xue L, Davis PB. Relationship between high-dose ibuprofen use and rate of decline in FEV1 among young patients with mild lung disease in the CFF Registry [abstract]. Pediatr Pulmonol 2004;27(Suppl):322. [Google Scholar]
- 5.Knudson RJ, Lebowitz MD, Holberg CJ, Burrows B. Changes in the normal maximal expiratory flow–volume curve with growth and aging. Am Rev Respir Dis 1983;127:725–734. [DOI] [PubMed] [Google Scholar]
- 6.Johnson CA, Butler SM, Konstan MW, Breen TJ, Morgan WJ. Estimating effectiveness in an observational study: a case study of dornase alfa in cystic fibrosis. J Pediatr 1999;134:734–739. [DOI] [PubMed] [Google Scholar]
- 7.Diggle P, Kenward MG. Informative dropout in longitudinal data analysis. Appl Stat 1994;43:49–93. [Google Scholar]
- 8.Verbecke G, Molenberghs G. Linear mixed models for longitudinal data. New York: Springer-Verlag; 2000.
- 9.Oermann CM, Sockrider MM, Konstan MW. The use of anti-inflammatory medications in cystic fibrosis: trends and physician attitudes. Chest 1999;115:1053–1058. [DOI] [PubMed] [Google Scholar]
- 10.Davis PB, Drumm M, Konstan MW. Cystic fibrosis: state of the art. Am J Respir Crit Care Med 1996;154:1229–1256. [DOI] [PubMed] [Google Scholar]
- 11.Bell EA, Grothe R, Zivkovich V, Foote JM, Wellendorf J. Pyloric channel stricture secondary to high-dose ibuprofen therapy in a patient with cystic fibrosis. Ann Pharmacother 1999;33:693–696. [DOI] [PubMed] [Google Scholar]
- 12.Mackey JE, Anbar RD. High-dose ibuprofen therapy associated with esophageal ulceration after pneumonectomy in a patient with cystic fibrosis: a case report. BMC Pediatr 2004;4:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kovesi TA, Swartz R, MacDonald N. Transient renal failure due to simultaneous ibuprofen and aminoglycoside therapy in children with cystic fibrosis. N Engl J Med 1998;338:65–66. [DOI] [PubMed] [Google Scholar]
- 14.Scott CS, Retsch-Bogart GZ, Henry MM. Renal failure and vestibular toxicity in an adolescent with cystic fibrosis receiving gentamicin and standard–dose ibuprofen. Pediatr Pulmonol 2001;31:314–316. [DOI] [PubMed] [Google Scholar]
- 15.Konstan MW, Hoppel CL, Chai B-L, Davis PB. Ibuprofen in children with cystic fibrosis: pharmacokinetics and adverse effects. J Pediatr 1991;118:956–964. [DOI] [PubMed] [Google Scholar]
- 16.Fennel PB, Quante J, Wilson K, Boyle M, Strunk R, Ferkol T. Use of high-dose ibuprofen in a pediatric cystic fibrosis center. J Cyst Fibros 2007;6:153–158. [DOI] [PubMed] [Google Scholar]
- 17.Davis PB, Byard PB, Konstan MW. Identifying treatments which halt progression of pulmonary disease in cystic fibrosis. Pediatr Res 1997;41:161–165. [DOI] [PubMed] [Google Scholar]
- 18.Morgan WJ, Butler SM, Johnson CA, Colin AA, FitzSimmons SC, Geller DE, Konstan MW, Light MJ, Rabin HR, Regelmann WE, et al.; Investigators and Coordinators of the Epidemiologic Study of Cystic Fibrosis. Epidemiologic study of cystic fibrosis: design and implementation of a prospective, multicenter, observational study of patients with cystic fibrosis in the US and Canada. Pediatr Pulmonol 1999;28:231–241. [DOI] [PubMed] [Google Scholar]
- 19.Konstan MW, Davis PB. Pharmacological approach for the discovery and development of new anti-inflammatory agents for the treatment of cystic fibrosis. Adv Drug Deliv Rev 2002;54:1409–1423. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.