Abstract
Long-term memory (LTM) formation usually requires repeated, spaced learning events and is achieved by the synthesis of specific proteins. Other memory forms require a single learning experience and are independent of protein synthesis. We investigated in two closely related parasitic wasp species, Cotesia glomerata and Cotesia rubecula, whether natural differences in foraging behaviour are correlated with differences in LTM acquisition and formation. These parasitic wasp species lay their eggs in young caterpillars of pierid butterflies and can learn to associate plant odours with a successful egg laying experience on caterpillars on the odour-producing plant. We used a classical conditioning set-up, while interfering with LTM formation through translation or transcription inhibitors. We show here that C. rubecula formed LTM after three spaced learning trials, whereas C. glomerata required only a single trial for LTM formation. After three spaced learning trials, LTM formation was complete within 4 h in C. glomerata, whereas in C. rubecula, LTM formation took 3 days. Linking neurobiology with ecology, we argue that this species-specific difference in LTM acquisition and formation is adaptive given the extreme differences in both the number of foraging decisions of the two wasp species and in the spatial distributions of their respective hosts in nature.
Keywords: learning, memory, evolution, foraging, Cotesia rubecula, Cotesia glomerata
1. Introduction
Learning and memory are remarkably similar traits across the Animal Kingdom, at the behavioural as well as at the cellular level (Dubnau 2003). In species ranging from snails and insects to mammals, memory is classified into temporally distinct forms. Short-term memory (STM) is labile and can be disrupted e.g. by anaesthesia applied shortly after learning (retrograde amnesia). This early memory phase is hence also called anaesthesia-sensitive memory. Hours after learning, memory is consolidated, i.e. solidified into less labile forms, which are resistant to retrograde amnesia. Two forms of consolidated memory can be distinguished of which long-term memory (LTM) requires gene expression and/or protein synthesis, whereas anaesthesia-resistant memory (ARM) does not (Margulies et al. 2005). LTM is normally formed after repeated learning events spaced by intervals (spaced learning) whereas a single event or a series of events immediately following each other (massed learning) induces ARM but is usually insufficient to induce LTM (Tully et al. 1994; Bailey et al. 1996; Menzel 2001).
Exceptions exist to the rule that spaced learning is required for LTM formation, e.g. appetitive conditioning in the snail Lymnaea (Fulton et al. 2005), fear conditioning in rats (Igaz et al. 2002) and oviposition learning in parasitic wasps (Collatz et al. 2006). Moreover, the numbers of learning events required for LTM acquisition can be increased or reduced by experimental changes in the expression of key genes involved in learning and memory formation such as the NMDA receptor (Tang et al. 1999) or CREB (Yin et al. 1995; Josselyn et al. 2001), and by artificial selection experiments (Mery & Kawecki 2002). These findings suggest that the number of learning events required for LTM is not due to a limitation of brain performance, but rather the result of the expression of inhibitory factors on the formation of memory (memory suppressor genes, Abel et al. 1998). The activity level of such memory suppressors determines the rate of learning; they prevent an animal from storage of information unless it has been proven to be reliable through repeated, confirmative experiences. Consolidation of new memories from STM into longer lasting memories can take hours to weeks, which again suggests an adaptation to enable the animal to evaluate acquired information with new experiences over an extended time window, before such information is stored into fixed neural substrates (Menzel 1999).
In ecological terms, the rate of learning and dynamics of memory formation are expected to be a function of their costs and benefits (Shettleworth 1993; Dukas 1999): a low rate is costly owing to suboptimal performance during learning (Laverty & Plowright 1988), whereas a high learning rate is costly owing to metabolic expenditure, i.e. acquisition and/or maintenance of memory (Mery & Kawecki 2005), and owing to the risk of storing irrelevant or misleading information. Factors such as life span, total number of lifetime experiences, variability of the environment and reliability of information are thought to influence the balance of costs and benefits of memory formation (Roitberg et al. 1993; Stephens 1993; Dukas 1998).
We have aimed to place current knowledge of learning and memory formation, obtained through research on model species such as the mouse and Drosophila, into an ecological framework by studying variation of learning rate and memory dynamics of parasitic wasps (Smid 2006). Learning in parasitic wasps has been extensively investigated and many species are known to learn to associate plant odours with the presence of suitable hosts during an oviposition experience on a plant (Lewis & Takasu 1990; Vet & Dicke 1992; Turlings et al. 1993; Vet et al. 1995; Steidle & van Loon 2003). Their naive preference for certain plant species, on which their hosts frequently occur, can be modified by oviposition experiences on other plant species. This learning task can be expected to be finely tuned to optimize the highly specialized foraging behaviour of the parasitoids. Such a model system is extremely useful to study-specific adaptations of learning and memory formation to ecological constraints. Indeed, some discrete differences were found in learning performance between parasitic wasp species (Poolman Simons et al. 1992; Potting et al. 1997; Geervliet et al. 1998; Bleeker et al. 2006; Tamo et al. 2006).
Our model system consists of Dutch strains of Cotesia glomerata and Cotesia rubecula, two closely related wasp species (Michel-Salzat & Whitfield 2004), which lay their eggs in young caterpillars of cabbage white butterflies, Pieris brassicae and Pieris rapae (figure 1). The gregarious C. glomerata lays up to 20 eggs into a single host and is considered a generalist; it can successfully develop in several host species, but mainly parasitizes the large white butterfly P. brassicae in The Netherlands (Geervliet et al. 2000). The solitary species C. rubecula lays a single egg into its host and is a specialist on the small cabbage white butterfly P. rapae. The two parasitic wasps C. glomerata and C. rubecula coexist in The Netherlands in different, but overlapping niches (Geervliet et al. 2000).
Figure 1.
Parasitic wasp (C. glomerata) laying eggs in a young caterpillar of P. brassicae. The encounters and subsequent oviposition in suitable hosts are strong reward stimuli in learning of parasitic wasps. Bar=3 mm.
Although their hosts occur on similar host plants, their searching strategy differs profoundly. This has been ascribed to the fact that both C. glomerata and its host P. brassicae are gregarious species whereas both C. rubecula and its host P. rapae are solitary species, resulting in an extreme difference in the number and temporal distribution of foraging experiences between the two wasp species (Bleeker et al. 2006). Cotesia glomerata has a lifetime fecundity of 500–2200 eggs (Vos & Vet 2004) and parasitizes many of the caterpillars that are present in clusters of up to 150 caterpillars (Lemasurier 1994), thus depositing up to half of its lifetime fecundity by finding only one infested host plant. The oviposition experiences occur in rapid sequence, which constitute one massed learning experience. In contrast, C. rubecula encounters only single hosts on a plant (Root & Kareiva 1984), lays only one egg per host, and has to find a new host plant for each new caterpillar host. Such a sequence of host encounters constitutes a series of many, temporally spaced learning experiences.
In addition, differences in host plant selection behaviour of the wasps' respective hosts, P. brassicae and P. rapae, results in a difference of the predictive value of an oviposition experience. Pieris brassicae deposits clusters of eggs on dense stands of plants of the same species (Lemasurier 1994). Finding a caterpillar of P. brassicae is therefore a reliable predictor that more caterpillars can be found on plants of the same species. Pieris rapae distributes its eggs over a wide area by depositing single eggs on isolated plants of different species (Root & Kareiva 1984). Finding a single host on a plant is not a reliable predictor for additional hosts on the same plant species. Thus, C. glomerata receives more reliable information from an encounter with its host P. brassicae, than C. rubecula from an encounter with P. rapae. This difference, in combination with the difference in the total number and temporal distribution of learning experiences, predicts that C. rubecula uses more host plant encounters and thereby learns at a lower rate, and takes more time to consolidate memory than C. glomerata. Previous research indeed suggested that C. glomerata is a ‘better’ learner than C. rubecula (Geervliet et al. 1998; Bleeker et al. 2006).
We here provide evidence that LTM formation is different between the two species, both in the number of required conditioning trials, and in the dynamics of memory consolidation. Using a strictly controlled classical conditioning set-up (Bleeker et al. 2006), that was identical for the two species, we trained naive wasps individually by giving them either one, three massed, or three spaced oviposition experiences on caterpillars of their respective hosts on a leaf of nasturtium, a plant that is unattractive to naive wasps. In order to inhibit the formation of LTM without affecting learning, formation of other memory forms and memory retrieval, wasps were fed anisomycin (ANI) or actinomycin D (ACD), a translation and transcription inhibitor, respectively. Memory retention was tested in a two-choice windtunnel test, during which wasps were allowed to fly upwind until landing on either a nasturtium plant or a cabbage plant, both infested with caterpillars of the wasp's respective hosts. When given the choice between cabbage and nasturtium, naive wasps of both species prefer cabbage, but the preference level for nasturtium can increase after learning such that nasturtium is even preferred over cabbage (Geervliet et al. 1998). Thus, the level of memory retention of a group of wasps can be expressed as the fraction of wasps in a group that land on the nasturtium plant compared with groups of naive wasps.
2. Material and methods
(a) Insects and plants
Cabbage plants (Brussels sprouts, Brassica oleracea var. gemmifera L. cv. Cyrus) and nasturtium plants (Tropaeolum majus L. cv. Glorious Gleam) were reared as described previously (Geervliet et al. 1998). We used plants of 3–4 weeks old, approximately 25 cm high, in an 11 cm black square pot. Two nasturtium plants were grown in each pot, whereas single cabbage plants were grown per pot to obtain a similar frontal density. Cotesia glomerata and C. rubecula (Hymenoptera: Braconidae) were obtained from colonies that originated from individuals collected in cabbage fields in the vicinity of Wageningen, The Netherlands, and were reared on P. brassicae L. and P. rapae L. (Lepidoptera: Pieridae), respectively, as described previously (Geervliet et al. 1998). Pieris larvae were reared on cabbage plants (B. oleracea) as described previously (Geervliet et al. 1998).
(b) Actinomycin D and anisomycin treatment and controls
Naive adult female wasps were used for training when they were 3–9 days old. In order to inhibit the formation of LTM without affecting learning, formation of other memory forms and memory retrieval, wasps were fed ANI or ACD, a translation and transcription inhibitor, respectively, in a sucrose solution before conditioning (Wustenberg et al. 1998; Watanabe et al. 2005; Collatz et al. 2006). Wasps were deprived of honey and water for 4 h and then offered 0.5 μl of a solution containing 0.1 mM ACD (Sigma) for C. glomerata and 0.5 mM ACD for C. rubecula in 2% sucrose (mean fresh body mass C. glomerata=1.4 mg; C. rubecula=1.8 mg). ANI (Sigma) was administered as described for ACD but at a concentration of 5 mM for C. glomerata and 25 mM for C. rubecula (see §3 for difference in concentration). Wasps were kept in 1.5 ml vials for 1 h after which the solution was found to be entirely consumed and then transferred to a glass cage with access to water and honey and given an oviposition experience within 2 h as described hereafter.
Groups of wasps serving as controls for drug-fed animals were always given the same treatment, except that the drug was omitted from the sucrose solution. Naive control groups were given the same drug treatment as the corresponding experienced groups. To asses toxic effects of ANI and ACD, mortality rates for female C. glomerata and C. rubecula wasps were determined. Each group of wasps was fed either sucrose, ANI or ACD as described above, and then kept in glass cages for 5 days. The number of dead wasps was determined each day (electronic supplementary material). This showed that deleterious effects of ACD and ANI became apparent after 3–4 days, depending on concentration. We chose concentrations that had a moderate effect on mortality rates, to ensure that effective concentrations were used without affecting responsiveness of the wasps. Note that the windtunnel test we used for assessing memory retention (see below) has an inherent control for responsiveness, as only wasps that show a proper flight response within 5 min of release were included in the data analysis. Statistical analysis (§ 2e) of the total response levels (i.e. the number of wasps that landed on either nasturtium or cabbage versus the total number of tested wasps), showed for C. glomerata that there was no effect of drug treatment and no interaction between drug treatment and conditioning (generalized linear modelling, GLM: conditioning: χ32=21.34, p<0.0001; treatment: χ22=1.31, p=0.52; conditioning×treatment: χ42=4.22, p=0.38). In the comparisons for specific contrasts, significant differences occurred only between naive wasps and each of the groups of conditioned wasps (not shown). For C. rubecula, there were effects of conditioning and treatment and an interaction between conditioning and treatment: (GLM: conditioning: χ32=64.11, p<0.0001; treatment: χ22=9.97, p=0.0069; conditioning×treatment: χ42=9.55, p=0.0085). In the comparisons for specific contrasts, significant differences were found only between naive wasps and each of the groups of conditioned wasps (not shown). Naive wasps had lower response levels compared with experienced wasps, which has been described before (Geervliet et al. 1998; Bleeker et al. 2006). There was no effect on response levels of either ACD or ANI compared with sucrose-fed wasps except for naive C. rubecula fed ANI, for which response levels were higher compared with the sucrose fed wasps (χ12=11.28, p=0.0008).
(c) Egg laying experience
For a more detailed description of the conditioning method and learning paradigm, we refer to Bleeker et al. (2006). Nasturtium plants were infested 24 h in advance with several freshly hatched caterpillars, to induce feeding damage. Naive female wasps were transferred from the breeding cage in a glass tube and allowed to walk to the open end of the tube. The tube was then brought towards the infested nasturtium leaf, ensuring that the antenna of the wasp contacted a caterpillar and its products. This stimulation induced an immediate oviposition response, lasting approximately 10 s. Meanwhile, the glass tube was held in front of the ovipositing wasp. After oviposition, the wasp typically walked in forward direction, into the glass tube again. The parasitized caterpillar was removed. The entire conditioning event was defined as a form of classical conditioning; the wasp was brought into the odour space of the nasturtium leaf (the conditioned stimulus) and was then rewarded by the unconditioned stimulus, the contact with host-derived substances (frass and silk spinning) followed by the actual oviposition. We consider this procedure to be distinct from operant conditioning, because the naturally occurring flight approach to the host plant was not included. The oviposition response is a reflex to contact with the host and host by-products, not a behavioural response to the plant odour. Furthermore, oviposition is not required for conditioning, these parasitic wasp species can learn to associate plant odours with suitable hosts when they encounter host by-products only (Geervliet et al. 1998). However, contact with host by-products followed by contact of the ovipositor with host haemolymph constitutes a stronger reward than contact with host by-products alone (Takasu & Lewis 2003). The conditioning sequence described above constitutes a single conditioning trial.
We used three different conditioning schedules: (i) single trial learning, (ii) three consecutive ovipositions in rapid sequence, without removing the wasp from the leaf in between (massed learning), or (iii) a sequence of three trials, spaced in time by a 10-min interval in a glass tube (three spaced trials). The interval of 10 min in between each trial was chosen based on studies of the effects of the intertrial interval on LTM formation in the honeybee (Gerber & Smith 1998; Eisenhardt 2006). These three conditioning procedures each represent a natural situation; a single trial conditioning when a wasp oviposits in a solitary caterpillar on a plant, a massed conditioning when a wasp oviposits on a cluster of gregarious caterpillars on a plant, and spaced conditioning when a wasp oviposits in caterpillars on different plants of the same species, spaced in time. Note that the wasp was not removed from the plant odour space in between the three ovipositions of the massed learning protocol, which is different from the more artificial massed conditioning protocols used in other studies on Drosophila or the honeybee (Apis mellifera) cited in this paper, but better reflects the natural situation of parasitic wasp learning. Cotesia glomerata females were offered P. brassicae caterpillars and C. rubecula females were offered P. rapae caterpillars. Wasps were grouped in glass cages and provided with water and honey. Cages were kept in a climate cabinet until the windtunnel assay was performed.
(d) Windtunnel assay
The response of the wasps to plant odours was tested in a windtunnel as described previously (Geervliet et al. 1998). One pot with a cabbage plant and one with a nasturtium plant of comparable size, were infested 24 h in advance on two leaves each with 20 freshly hatched caterpillars. For tests with C. glomerata, plants were infested with P. brassicae, and for C. rubecula with P. rapae. One infested cabbage plant and one infested nasturtium plant were placed upwind in the windtunnel with approximately 10 cm distance between the leaves. Each wasp was released on a platform 70 cm downwind from the two plants. Wasps that initiated flight within 5 min and made a first landing on one of the two plants were scored as showing a response; all other wasps were scored as having shown no response. Wasps were tested only once in the windtunnel and then discarded. The positions of cabbage and nasturtium plants were alternated after each fifth wasp tested. The time after conditioning is measured in hours, from the end of the first conditioning trial. For each data point, results were collected from at least three different days with some exceptions (see electronic supplementary material, table 1), using different plants and at least 10 wasps for each experimental day.
(e) Statistical analysis
We used GLM procedures using procedure Genmod in SAS v. 8.02 (Proc Genmod, SAS, Inc., Chicago, IL) for data with a binomial distribution of error variance and a logit-link function. When the choice distributions of wasps were compared, the fraction of individuals landing on nasturtium in the windtunnel was used as the response variable with number of responding wasps as the binomial total. When the response levels of parasitoids were compared, the fraction of individuals that made a choice was the response variable with all the parasitoids released in the bioassay as the binomial total. Data collected on different experimental days were considered as replicates. In case of overdispersal, we allowed the variance functions of the binomial distribution to have a multiplicative overdispersion factor (DSCALE option) by dividing the square root of the deviance of the model by the degrees of freedom (McCullagh & Nelder 1989). The treatments tested in each experiment are given below. When main effects or their interactions were found significantly different, further separation of the treatment levels was carried out by acquiring specific contrasts for particular comparisons. For these contrasts, p values are given in the figures.
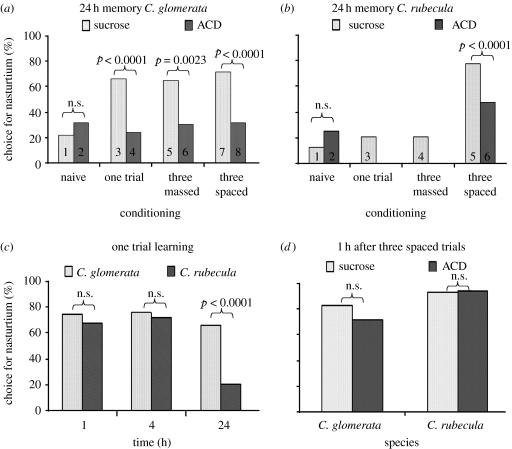
In experiment 1a (figure 2a,b), we tested for C. glomerata whether conditioning (four levels: naive, single trial, three massed trials and three spaced trials), and inhibitor treatment (two levels: sucrose and sucrose+ACD) had an effect on the response level to the nasturtium plant (GLM: conditioning: χ32=15.01, p<0.0018; treatment: χ12=26.79, p<0.0001; conditioning×treatment: χ32=20.36, p<0.0001) For C. rubecula (figure 2b), we tested whether conditioning (four levels: naive, single trial, three massed trials and three spaced trials, and inhibitor treatment (two levels: sucrose and sucrose+ACD) had an effect on the response level to the nasturtium plant (GLM: conditioning: χ32=58.13, p<0.0001; treatment: χ12=0.46, p<0.50; conditioning×treatment: χ12=9.26, p<0.0023).
Figure 2.
Memory retention levels for C. glomerata and C. rubecula and the effects of ACD. (a) C. glomerata and (b) C. rubecula, 24 h after one trial, three massed and three spaced trials. Naive wasps have a low choice level for nasturtium, whereas after a single or three massed trials, choice for nasturtium is increased in C. glomerata, but not in C. rubecula. After three spaced trials, both species show a similar increase in choice for nasturtium. The choice increase is inhibited by treatment with ACD to levels similar as naive control wasps in C. glomerata, but the inhibition results in a choice for nasturtium still higher than naive controls in C. rubecula. The p values of specific contrasts between treatments, as numbered in corresponding bars are, (a) contrasts 1–3, 1–5 and 1–7: p<0.0001, contrasts 2–4, 2–6 and 2–8 are not significant; (b) contrasts 1–3 and 1–4 are not significant, contrast 1–5: p<0.0001; (c) contrast 2–6: p=0.058. Time dependency of memory retention after one trial learning up to 24 h. Memory retention is initially similar, but decays in C. rubecula between 4 and 24 h, whereas it remains stable in C. glomerata. (d) Memory retention after three spaced trials, measured after 1 h. For both wasp species, retention levels are not affected by ACD treatment, showing that ACD treatment does not influence the process of learning.
For control experiment 1b (figure 2c, 1 trial learning at different retention times), we tested whether time (three levels: 1, 4 and 24 h) and species (two levels: C. glomerata and C. rubecula) had an effect on the response level to the nasturtium plant (GLM: time: χ22=28.16, p<0.0001; species: χ12=14.12, p=0.0002; time×species: χ22=12.14, p=0.0023).
For control experiment 1c (figure 2d; three spaced trials tested 1 h after conditioning), we tested whether treatment (two levels: sucrose and sucrose+ACD) and species (two levels: C. glomerata and C. rubecula) had an effect on the response level to the nasturtium plant. (GLM: species: χ12=9.12; treatment: χ12=0.19, p=0.67; species×treatment: χ12=0.93, p=0.34.)
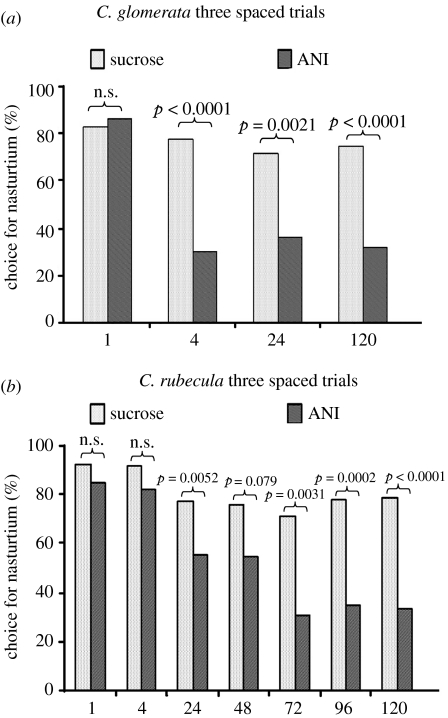
In experiment 2 (figure 3a,b), we tested whether time (C. glomerata, four levels: 1, 4, 24 and 120 h; C. rubecula, seven levels: 1, 4, 24, 48, 72, 96 and 120 h) and treatment (two levels: sucrose and sucrose+ANI) had effects on response level to nasturtium plants (GLM: C. glomerata, treatment: χ12=28.05, p<0.0001; time: χ32=24.08, p≤0.0001; treatment×time: χ32=12.10, p=0.007. C. rubecula, treatment: χ12=37.92, p<0.0001; time: χ62=49.25, p<0.0001; treatment×time: χ62=6.34, p=0.39). As a control for the effect of ANI on preference for nasturtium, we tested whether treatment (two levels: sucrose and sucrose+ANI) had an effect on the response level to the nasturtium plant of naive wasps, measured 24 h after treatment (GLM: C. glomerata: χ12=1.05, p=0.31; C. rubecula: χ12=0.047, p=0.83).
Figure 3.
Memory retention after spaced training in (a) C. glomerata and (b) C. rubecula. Wasps were fed either 0.5 μl sucrose or 0.5 μl sucrose+translation inhibitor ANI prior to learning. Both species show stable memory levels within the tested interval of 5 days. ANI treatment has no effect 1 h after learning. In C. glomerata, 4 h and later memory is strongly inhibited by ANI, and the level of this inhibition does not increase over time. In C. rubecula, inhibitory effects of ANI become apparent at 24 h, and maximum inhibition is reached after 72 h. The specific contrast between the ANI-treated groups of 24 and 120 h memory retention was significant (p=0.035).
As a control for the effect of conditioning and treatment on the total response level (wasps in a group that land either on cabbage or nasturtium versus the total wasps tested), we tested whether conditioning (four levels: naive, single trial, three massed trials and three spaced trials) and treatment (three levels: sucrose, sucrose+ACD and sucrose+ANI) had an effect on the response variable, which was the total number of responding wasps, whereas the number of wasps tested was used as binomial total (GLM: C. glomerata: conditioning: χ32=21.34, p<0.0001; treatment: χ22=1.31, p=0.52; conditioning×treatment: χ42=4.22, p=0.38. C. rubecula: conditioning: χ32=64.11, p<0.0001; treatment: χ22=9.97, p=0.0069; conditioning×treatment: χ42=9.55, p=0.0085).
3. Results
All raw data from experiments 1 and 2, as described below, are listed as online supplementary data. For p values of specific contrasts between particular comparisons, resulting from GLM analysis, see corresponding figures. In experiment 1a, we measured 24 h memory retention after a single, massed or spaced conditioning schedules (figure 2a,b). Wasps were treated individually by feeding the transcription inhibitor ACD in sucrose, or sucrose alone to measure the transcription-dependent component of the observed memory levels. Naive wasps of both species fed sucrose had a low response level for nasturtium, and this level is not changed when naive wasps were fed sucrose+ACD (figure 2a,b). In sucrose-fed C. glomerata, a single trial resulted in strong 24 h memory retention when compared with naive wasps. When ACD-fed wasps were given a single conditioning trial, 24 h memory was reduced to a level not different from naive, ACD-fed wasps, showing that 24 h memory was composed entirely of a transcription-dependent memory component. In sucrose-fed C. rubecula, 24 h memory retention after a single conditioning trial was not different from naive, sucrose fed wasps. This showed that C. rubecula either did not learn from a single trial or that only a short-lasting memory was formed. To test this possibility, we measured memory retention also at 1 and 4 h after a single trial (experiment 1b, figure 2c). This showed that 1 and 4 h memory retention reached similar levels in both species. This shows that C. rubecula forms memory for nasturtium after a single trial, but it lasts for a shorter time than in C. glomerata.
A massed learning protocol (three oviposition experiences on nasturtium in quick succession) yielded the same differences in 24 h memory retention levels as single trial learning. For C. glomerata, massed learning gave a strong 24 h memory compared with naive wasps and a reduction of this memory by ACD treatment to a level not different from naive, ACD-fed wasps (figure 2a). Cotesia rubecula did not show any 24 h memory retention, as there was no difference with naive wasps (figure 2b).
A spaced learning protocol (three trials with a 10 min interval) yielded a strong 24 h memory for both species. In C. glomerata, this memory was inhibited in ACD-fed wasps to a level not different from naive, ACD-fed wasps. In C. rubecula, memory was also inhibited by ACD but the difference with naive, ACD-fed wasps, was still marginally significant (p=0.058), even though we used a higher concentration of ACD than for C. glomerata (0.5 versus 0.1 mM). The inhibition of 24 h memory retention is not due to interference of ACD treatment with learning because control experiments measuring 1 h retention after three spaced trials show that retention levels are not affected by ACD in either species (experiment 1c, figure 2d). Thus, both wasp species have a high 24 h memory retention which is entirely transcription-dependent in C. glomerata, whereas it is partially transcription-dependent in C. rubecula. An explanation for this partial inhibition could be that the effect of ACD in C. rubecula is only moderate. This explanation is, however, unlikely given the fact that a five times lower concentration of this drug leads to complete inhibition of 24 h memory in C. glomerata.
In experiment 2, we confirmed the observed difference in transcription dependency of spaced learning-induced 24 h memory of experiment 1, and tested whether it is caused by differences in temporal dynamics of LTM consolidation. Furthermore, we tested the possibility that the transcription-independent memory component in C. rubecula may represent a translation-dependent form of LTM. Such a difference in LTM forms has been described for the honeybee (Eisenhardt 2006). Individual wasps were fed translation inhibitor ANI in a sucrose solution as inhibitor of protein synthesis-dependent memory formation and controls were fed sucrose alone. Wasps were tested for memory retention at intervals between 1 and 120 h after learning. Since pilot experiments indicated that 24 h memory retention was inhibited only partially by ANI in C. rubecula compared with C. glomerata, we used a five times higher concentration of ANI for C. rubecula (25 versus 5 mM) to eliminate the possibility that this partial inhibition was caused by a lower sensitivity to ANI in C. rubecula. There was no difference in preference levels of naive wasps between sucrose-fed wasps and sucrose+ANI-fed wasp (not shown, specific contrasts for C. glomerata, p=0.31; C. rubecula, p=0.83). In both species, sucrose-fed wasps that received three spaced trials had a stable memory lasting 5 days (figure 3). There was, however, a profound difference in the temporal dynamics of ANI inhibition between the species. In C. glomerata, memory performance of ANI-fed wasps was not different from sucrose-fed wasps after 1 h, but maximum inhibition was reached and remained stable after 4 h. This shows that 1 h retention was not affected by ANI, and that consolidation of a protein synthesis-dependent memory was complete after 4 h. In C. rubecula, 1 and 4 h memory retention in ANI-fed wasps was not different from sucrose fed wasps, showing that consolidation of a protein synthesis-dependent memory component had not started at that time. At 24 h, ANI-fed C. rubecula had a lower memory retention than sucrose fed wasps, but still higher than ANI-fed wasps measured at 120 h. To reveal the dynamics of the consolidation of the ANI-dependent memory trace, we measured memory retention for C. rubecula at 24 h intervals till 120 h. This showed that the ANI-dependent component of the observed memory gradually reaches its maximum level at 72 h and remains stable thereafter. Thus, a protein synthesis-independent memory trace coexists with a protein synthesis-dependent memory trace in C. rubecula at 24 h, whereas memory is entirely protein synthesis-dependent at 72 h.
4. Discussion
Our results show that C. glomerata forms protein synthesis-dependent LTM after only a single conditioning trial whereas C. rubecula needs three spaced trials. There was also a difference in consolidation dynamics of LTM; in C. glomerata consolidation was complete within 4 h, whereas this process took 2–3 days in C. rubecula. This observation is in line with our expectation that C. rubecula uses more experiences and more time to evaluate information before such information is stored in LTM. In addition, and as a consequence of the extreme difference in LTM consolidation dynamics, we found that a protein synthesis-independent memory trace, presumably ARM, is present up to 48 h after spaced learning in C. rubecula, whereas the memory trace is built up exclusively by protein synthesis-dependent memory in C. glomerata. There is no difference between the effects of ACD and ANI at 24 h in both wasp species, showing that the LTM component is transcription-dependent. This is the first demonstration of natural differences in quantity and quality of learning events required for LTM formation between closely related species, which correlates to their specific ecological constraints.
The situation in C. rubecula, where two different memory traces seem to coexist up to 3 days, is similar to that described for Drosophila (Tully et al. 1994), where 1 day memory after 10 spaced trials consists of a protein synthesis-dependent component and a protein synthesis-independent form of consolidated memory, ARM. By this concept, ARM is induced in Drosophila by both single and massed training, whereas both LTM and ARM are induced by spaced training (Tully et al. 1994). This concept of LTM and ARM coexisting in parallel in Drosophila was recently challenged (Isabel et al. 2004) based on the results of a study on a subpopulation of the Drosophila mutant ala, which lacks the vertical (alpha) lobes of the mushroom bodies in the brain, and is incapable of LTM formation, but has fully functional ARM (Pascual & Preat 2001). This mutant has normal 1 day retention after single trial learning, whereas 10 spaced trials result in a complete loss of 1 day memory retention. The conclusion from this experiment that LTM and ARM are consolidated exclusively (Isabel et al. 2004) is controversial given the results of other studies (Margulies et al. 2005), but interesting in the light of our results, which suggest that exclusive consolidation of LTM occurs in C. glomerata but in parallel with ARM in C. rubecula. Apparently, both parallel ARM–LTM and exclusive LTM consolidation can be a feasible way of long-lasting memory consolidation. We currently study the dynamics of consolidation of STM into ARM and/or LTM in our wasp species using retrograde amnesia to investigate this phenomenon further.
An extreme difference exists between C. glomerata and C. rubecula in the number of oviposition experiences, because C. glomerata as well as its host P. brassicae are gregarious, whereas C. rubecula and its host P. rapae are solitary species. This causes the oviposition experiences in the natural situation to occur as many series of spaced learning experiences in the case of C. rubecula, and as a few massed learning experiences in the case of C. glomerata, and explains why C. rubecula can spend series of learning experiences before it stores information as LTM, whereas C. glomerata needs to learn from one massed experience on a single encounter with a host plant. In addition, a longer consolidation time for LTM provides C. rubecula with a longer time window for evaluation of multiple experiences. Recent results in Drosophila show that energetic costs for LTM acquisition and/or consolidation are considerable, in sharp contrast with the costs of ARM (Mery & Kawecki 2005), suggesting that ARM can be interpreted as a form of low-cost, long-lasting memory. This provides an additional explanation for the difference in exclusive and parallel ARM/LTM consolidation; single trial LTM consolidation would represent high-energy expenditure for C. rubecula, with its high number of spaced learning experiences, but not for C. glomerata, with its few massed learning experiences.
Our species comparison is the first study to provide evidence for species-specific memory dynamics, whereby variation in LTM acquisition correlates with a natural difference in the animal's behavioural ecology. Our parasitic wasp model provides a clear-cut species-specific difference in cognitive performance that can be determined in a standardized laboratory assay, which is to our knowledge a unique feature in the study of learning and memory.
Acknowledgments
We thank Leo Koopman, André Gidding and Frans van Aggelen for rearing of insects, Gerrit Gort for advice on the statistical analysis and two anonymous referees, Gene Robinson, Marcel Dicke and Ties Huigens for comments on this manuscript. This work was supported by grant no. 2003836030 from the Chinese Scholarship Council to G.W., and grant no. 810.34.002 from the Dutch organization for scientific research NWO-ALW to M.B.
Supplementary Material
Included in file
References
- Abel T, Martin K.C, Bartsch D, Kandel E.R. Memory suppressor genes: inhibitory constraints on the storage of long-term memory. Science. 1998;279:338–341. doi: 10.1126/science.279.5349.338. doi:10.1126/science.279.5349.338 [DOI] [PubMed] [Google Scholar]
- Bailey C.H, Bartsch D, Kandel E.R. Toward a molecular definition of long-term memory storage. Proc. Natl Acad. Sci. USA. 1996;93:13 445–13 452. doi: 10.1073/pnas.93.24.13445. doi:10.1073/pnas.93.24.13445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bleeker M.A.K, Smid H.M, Steidle J.L.M, Kruidhof M, van Loon J.J.A, Vet L.E.M. Differences in memory dynamics between two closely related parasitoid wasp species. Anim. Behav. 2006;71:1343–1350. doi:10.1016/j.anbehav.2005.09.016 [Google Scholar]
- Collatz J, Muller C, Steidle J.L.M. Protein synthesis-dependent long-term memory induced by one single associative training trial in the parasitic wasp Lariophagus distinguendus. Learn. Mem. (Cold Spring Harb.) 2006;13:263–266. doi: 10.1101/lm.192506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubnau J. Neurogenetic dissection of conditioned behavior: evolution by analogy or homology? J. Neurogenet. 2003;17:295–326. doi: 10.1080/01677060390441859. doi:10.1080/01677060390441859 [DOI] [PubMed] [Google Scholar]
- Dukas R. Constraints on information processing and their effects on behavior. In: Dukas R, editor. Cognitive ecology: the evolutionary ecology of information processing and decision making. University of Chicago Press; Chicago, IL; London, UK: 1998. pp. 89–119. [Google Scholar]
- Dukas R. Costs of memory: ideas and predictions. J. Theor. Biol. 1999;197:41–50. doi: 10.1006/jtbi.1998.0856. doi:10.1006/jtbi.1998.0856 [DOI] [PubMed] [Google Scholar]
- Eisenhardt D. Learning and memory formation in the honeybee (Apis mellifera) and its dependency on the cAMP-protein kinase A pathway. Anim. Biol. 2006;56:259–278. doi:10.1163/157075606777304249 [Google Scholar]
- Fulton D, Kemenes I, Andrew R.J, Benjamin P.R. A single time-window for protein synthesis-dependent long-term memory formation after one-trial appetitive conditioning. Eur. J. Neurosci. 2005;21:1347–1358. doi: 10.1111/j.1460-9568.2005.03970.x. doi:10.1111/j.1460-9568.2005.03970.x [DOI] [PubMed] [Google Scholar]
- Geervliet J.B.F, Vreugdenhil A.I, Dicke M, Vet L.E.M. Learning to discriminate between infochemicals from different plant–host complexes by the parasitoids Cotesia glomerata and C. rubecula. Entomol. Exp. Appl. 1998;86:241–252. doi:10.1023/A:1003186706517 [Google Scholar]
- Geervliet J.B.F, Verdel M.S.W, Snellen H, Schaub J, Dicke M, Vet L.E.M. Coexistence and niche segregation by field populations of the parasitoids Cotesia glomerata and C. rubecula in The Netherlands: predicting field performance from laboratory data. Oecologia. 2000;124:55–63. doi: 10.1007/s004420050024. doi:10.1007/s004420050024 [DOI] [PubMed] [Google Scholar]
- Gerber B, Smith B.H. Visual modulation of olfactory learning in honeybees. J. Exp. Biol. 1998;201:2213–2217. doi: 10.1242/jeb.201.14.2213. [DOI] [PubMed] [Google Scholar]
- Igaz L.M, Vianna M.R.M, Medina J.H, Izquierdo I. Two time periods of hippocampal mRNA synthesis are required for memory consolidation of fear-motivated learning. J. Neurosci. 2002;22:6781–6789. doi: 10.1523/JNEUROSCI.22-15-06781.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isabel G, Pascual A, Preat T. Exclusive consolidated memory phases in Drosophila. Science. 2004;304:1024–1027. doi: 10.1126/science.1094932. doi:10.1126/science.1094932 [DOI] [PubMed] [Google Scholar]
- Josselyn S.A, Shi C, Carlezon W.A, Jr, Neve R.L, Nestler E.J, Davis M. Long-term memory is facilitated by cAMP response element-binding protein overexpression in the amygdala. J. Neurosci. 2001;21:2404–2412. doi: 10.1523/JNEUROSCI.21-07-02404.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laverty T.M, Plowright R.C. Flower handling by bumblebees—a comparison of specialists and generalists. Anim. Behav. 1988;36:733–740. doi:10.1016/S0003-3472(88)80156-8 [Google Scholar]
- Lemasurier A.D. Costs and benefits of egg clustering in Pieris brassicae. J. Anim. Ecol. 1994;63:677–685. doi:10.2307/5233 [Google Scholar]
- Lewis W.J, Takasu K. Use of learned odours by a parasitic wasp in accordance with host and food needs. Nature. 1990;348:635–636. doi:10.1038/348635a0 [Google Scholar]
- Margulies C, Tully T, Dubnau J. Deconstructing memory in Drosophila. Curr. Biol. 2005;15:R700–R713. doi: 10.1016/j.cub.2005.08.024. doi:10.1016/j.cub.2005.08.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCullagh P, Nelder J.A. Chapman & Hall; London, UK: 1989. Generalized linear models. [Google Scholar]
- Menzel R. Memory dynamics in the honeybee. J. Comp. Physiol. A. 1999;185:323–340. doi:10.1007/s003590050392 [Google Scholar]
- Menzel R. Searching for the memory trace in a mini-brain, the honeybee. Learn. Mem. (Cold Spring Harb.) 2001;8:53–62. doi: 10.1101/lm.38801. [DOI] [PubMed] [Google Scholar]
- Mery F, Kawecki T.J. Experimental evolution of learning ability in fruit flies. Proc. Natl Acad. Sci. USA. 2002;99:14 274–14 279. doi: 10.1073/pnas.222371199. doi:10.1073/pnas.222371199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mery F, Kawecki T.J. A cost of long-term memory in Drosophila. Science. 2005;308:1148. doi: 10.1126/science.1111331. doi:10.1126/science.1111331 [DOI] [PubMed] [Google Scholar]
- Michel-Salzat A, Whitfield J.B. Preliminary evolutionary relationships within the parasitoid wasp genus Cotesia (Hymenoptera : Braconidae : Microgastrinae): combined analysis of four genes. Syst. Entomol. 2004;29:371–382. doi:10.1111/j.0307-6970.2004.00246.x [Google Scholar]
- Pascual A, Preat T. Localization of long-term memory within the Drosophila mushroom body. Science. 2001;294:1115–1117. doi: 10.1126/science.1064200. doi:10.1126/science.1064200 [DOI] [PubMed] [Google Scholar]
- Poolman Simons M.T.T, Suverkropp B.P, Vet L.E.M, De Moed G. Comparison of learning in related generalist and specialist eucoilid parasitoids. Entomol. Exp. Appl. 1992;64:117–124. [Google Scholar]
- Potting R.P.J, Otten H, Vet L.E.M. Absence of odour learning in the stemborer parasitoid Cotesia flavipes. Anim. Behav. 1997;53:1211–1223. doi: 10.1006/anbe.1996.0382. doi:10.1006/anbe.1996.0382 [DOI] [PubMed] [Google Scholar]
- Roitberg B.D, Reid M.L, Li C. Choosing hosts and mates, the value of learning. In: Papaj D.R, Lewis A.C, editors. Insect learning: ecological and evolutionary perspectives. Chapman & Hall; New York, NY; London, UK: 1993. pp. 174–194. [Google Scholar]
- Root R.B, Kareiva P.M. The search for resources by cabbage butterflies (Pieris rapae)—ecological consequences and adaptive significance of Markovian movements in a patchy environment. Ecology. 1984;65:147–165. doi:10.2307/1939467 [Google Scholar]
- Shettleworth S.J. Varieties of learning and memory in animals. J. Exp. Psychol. Anim. Behav. Process. 1993;19:5–14. doi: 10.1037//0097-7403.19.1.5. doi:10.1037/0097-7403.19.1.5 [DOI] [PubMed] [Google Scholar]
- Smid, H. M. 2066 Variation in learning of herbivory-induced plant odours by parasitic wasps: from brain to behaviour. In Chemical ecology: from gene to ecosystem vol. 16 (eds. M. Dicke & W. Takken), Wageningen University frontis series, pp. 89–105. Dordrecht, The Netherlands: Springer.
- Steidle J.L.M, van Loon J.J.A. Dietary specialization and infochemical use in carnivorous arthropods: testing a concept. Entomol. Exp. Appl. 2003;108:133–148. doi:10.1046/j.1570-7458.2003.00080.x [Google Scholar]
- Stephens D.W. Learning and behavioral ecology: incomplete information and environmental predictability. In: Papaj D.R, Lewis A.C, editors. Insect learning. Chapman & Hall; New York, NY; London, UK: 1993. pp. 195–218. [Google Scholar]
- Takasu K, Lewis W.J. Learning of host searching cues by the larval parasitoid Microplitis croceipes. Entomol. Exp. Appl. 2003;108:77–86. doi:10.1046/j.1570-7458.2003.00070.x [Google Scholar]
- Tamo C, Ricard I, Held M, Davison A.C, Turlings T.C.J. A comparison of naive and conditioned responses of three generalist endoparasitoids of lepidopteran larvae to host-induced plant odours. Anim. Biol. 2006;56:205–220. [Google Scholar]
- Tang Y.P, Shimizu E, Dube G.R, Rampon C, Kerchner G.A, Zhuo M, Liu G.S, Tsien J.Z. Genetic enhancement of learning and memory in mice. Nature. 1999;401:63–69. doi: 10.1038/43432. doi:10.1038/43432 [DOI] [PubMed] [Google Scholar]
- Tully T, Preat T, Boynton S.C, Del Vecchio M. Genetic dissection of consolidated memory in Drosophila. Cell. 1994;79:35–47. doi: 10.1016/0092-8674(94)90398-0. doi:10.1016/0092-8674(94)90398-0 [DOI] [PubMed] [Google Scholar]
- Turlings T.C.J, Wäckers F.L, Vet L.E.M, Lewis W.J, Tumlinson J.H. Learning of host-finding cues by hymenopterous parasitoids. In: Papaj D.R, Lewis A.C, editors. Insect learning: ecological and evolutionary perspectives. Chapman & Hall; New York, NY; London, UK: 1993. pp. 51–78. [Google Scholar]
- Vet L.E.M, Dicke M. Ecology of infochemical use by natural enemies in a tritrophic context. Annu. Rev. Entomol. 1992;37:141–172. doi:10.1146/annurev.en.37.010192.001041 [Google Scholar]
- Vet L.E.M, Lewis W.J, Cardé R.T. Parasitoid foraging and learning. In: Cardé R.T, Bell W.J, editors. Chemical ecology of insects. Chapman & Hall; New York, NY: 1995. pp. 65–101. [Google Scholar]
- Vos M, Vet L.E.M. Geographic variation in host acceptance by an insect parasitoid: genotype versus experience. Evol. Ecol. Res. 2004;6:1021–1035. [Google Scholar]
- Watanabe H, Takaya T, Shimoi T, Ogawa H, Kitamura Y, Oka K. Influence of mRNA and protein synthesis inhibitors on the long-term memory acquisition of classically conditioned earthworms. Neurobiol. Learn. Mem. 2005;83:151–157. doi: 10.1016/j.nlm.2004.11.003. doi:10.1016/j.nlm.2004.11.003 [DOI] [PubMed] [Google Scholar]
- Wustenberg D, Gerber B, Menzel R. Long- but not medium-term retention of olfactory memory in honeybees is impaired by actinomycin D and anisomycin. Eur. J. Neurosci. 1998;10:2742–2745. doi: 10.1046/j.1460-9568.1998.00319.x. doi:10.1046/j.1460-9568.1998.00319.x [DOI] [PubMed] [Google Scholar]
- Yin J.C, Del Vecchio M, Zhou H, Tully T. CREB as a memory modulator: induced expression of a dCREB2 activator isoform enhances long-term memory in Drosophila. Cell. 1995;81:107–115. doi: 10.1016/0092-8674(95)90375-5. doi:10.1016/0092-8674(95)90375-5 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Included in file