Abstract
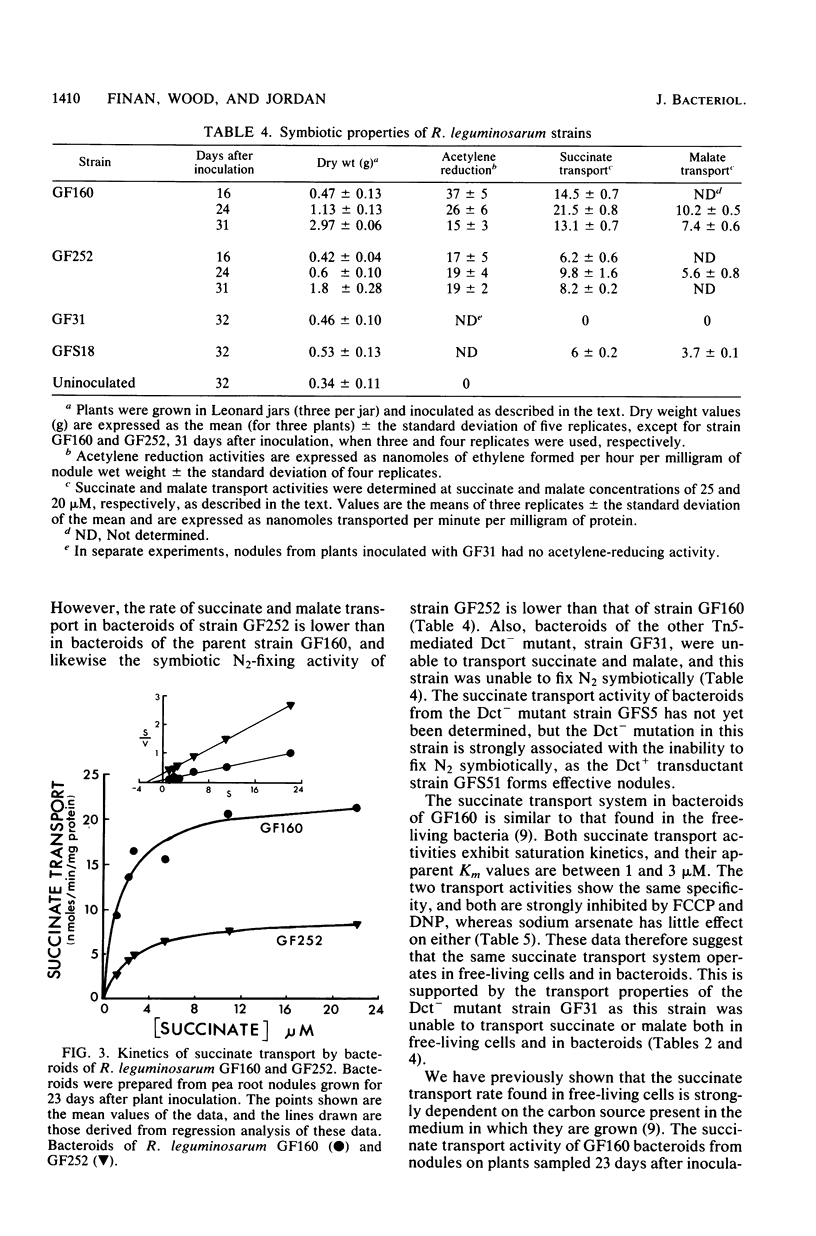
The transport of succinate was studied in bacteroids of an effective, streptomycin-resistant strain (GF160) of Rhizobium leguminosarum. High levels of succinate transport occurred, and the kinetics, specificity, and sensitivity to metabolic inhibitors were similar to those previously described for free-living cells. The symbiotic properties of two transposon (Tn5)-mediated C4-dicarboxylate transport mutants (strains GF31 and GF252) were determined. Strain GF31 formed ineffective nodules, and bacteroids from these nodules showed no succinate transport activity. Strain GF252 formed partially effective nodules, and bacteroids from these nodules showed about 50% of the succinate transport activity of the parent bacteroids. Another dicarboxylic acid transport mutant (Dct-), strain GFS5, isolated after N-methyl-N'-nitro-N-nitrosoguanidine mutagenesis, formed ineffective nodules. The ability to form ineffective nodules in strains GF31 and GFS5 was shown to correlate with the Dct- phenotype. The data indicate that the presence of a functional C4-dicarboxylic acid transport system is essential for N2 fixation to occur in pea nodules.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Avissar Y. J., Nadler K. D. Stimulation of tetrapyrrole formation in Rhizobium japonicum by restricted aeration. J Bacteriol. 1978 Sep;135(3):782–789. doi: 10.1128/jb.135.3.782-789.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergersen J. F., Turner G. L. Nitrogen fixation by the bacteroid fraction of breis of soybean root nodules. Biochim Biophys Acta. 1967 Aug 29;141(3):507–515. doi: 10.1016/0304-4165(67)90179-1. [DOI] [PubMed] [Google Scholar]
- Beringer J. E. R factor transfer in Rhizobium leguminosarum. J Gen Microbiol. 1974 Sep;84(1):188–198. doi: 10.1099/00221287-84-1-188. [DOI] [PubMed] [Google Scholar]
- Blasco J. A., Jordan D. C. Nitrogen fixation in the muskeg ecosystem of the James Bay Lowlands, Northern Ontario. Can J Microbiol. 1976 Jul;22(7):897–907. doi: 10.1139/m76-130. [DOI] [PubMed] [Google Scholar]
- Cutting J. A., Schulman H. M. The site of heme synthesis in soybean root nodules. Biochim Biophys Acta. 1969 Dec 30;192(3):486–493. doi: 10.1016/0304-4165(69)90398-5. [DOI] [PubMed] [Google Scholar]
- Duncan M. J., Fraenkel D. G. alpha-Ketoglutarate dehydrogenase mutant of Rhizobium meliloti. J Bacteriol. 1979 Jan;137(1):415–419. doi: 10.1128/jb.137.1.415-419.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finan T. M., Wood J. M., Jordan D. C. Succinate transport in Rhizobium leguminosarum. J Bacteriol. 1981 Oct;148(1):193–202. doi: 10.1128/jb.148.1.193-202.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godfrey C. A., Dilworth M. J. Haem biosynthesis from ( 14 C)- -aminolaevulinic acid in laboratory-grown and root nodule Rhizobium lupini. J Gen Microbiol. 1971 Dec;69(3):385–390. doi: 10.1099/00221287-69-3-385. [DOI] [PubMed] [Google Scholar]
- Johnson G. V., Evans H. J., Ching T. Enzymes of the glyoxylate cycle in rhizobia and nodules of legumes. Plant Physiol. 1966 Oct;41(8):1330–1336. doi: 10.1104/pp.41.8.1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laane C., Haaker H., Veeger C. Involvement of the cytoplasmic membrane in nitrogen fixation by Rhizobium leguminosarum bacteroids. Eur J Biochem. 1978 Jun 1;87(1):147–153. doi: 10.1111/j.1432-1033.1978.tb12361.x. [DOI] [PubMed] [Google Scholar]
- Leonard L. T. A Simple Assembly for Use in the Testing of Cultures of Rhizobia. J Bacteriol. 1943 Jun;45(6):523–527. doi: 10.1128/jb.45.6.523-527.1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo T. C., Sanwal B. D. Isolation of the soluble substrate recognition component of the dicarboxylate transport system of Escherichia coli. J Biol Chem. 1975 Feb 25;250(4):1600–1602. [PubMed] [Google Scholar]
- Muecke P. S., Wiskich J. T. Respiratory activity of mitochondria from legume root nodules. Nature. 1969 Feb 15;221(5181):674–675. doi: 10.1038/221674a0. [DOI] [PubMed] [Google Scholar]
- Nadler K. D., Avissar Y. J. Heme Synthesis in Soybean Root Nodules: I. On the Role of Bacteroid delta-Aminolevulinic Acid Synthase and delta-Aminolevulinic Acid Dehydrase in the Synthesis of the Heme of Leghemoglobin. Plant Physiol. 1977 Sep;60(3):433–436. doi: 10.1104/pp.60.3.433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips M. L., Williams G. R. Anion transporters in plant mitochondria. Plant Physiol. 1973 Apr;51(4):667–670. doi: 10.1104/pp.51.4.667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips M. L., Williams G. R. Effects of 2-Butylmalonate, 2-Phenylsuccinate, Benzylmalonate, and p-Iodobenzylmalonate on the Oxidation of Substrates by Mung Bean Mitochondria. Plant Physiol. 1973 Feb;51(2):225–228. doi: 10.1104/pp.51.2.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quagliariello E., Palmieri F., Prezioso G., Klingenberg M. Kinetics of succinate uptake by rat-liver mitochondria. FEBS Lett. 1969 Aug;4(4):251–254. doi: 10.1016/0014-5793(69)80247-4. [DOI] [PubMed] [Google Scholar]
- REYNOLDS E. S. The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J Cell Biol. 1963 Apr;17:208–212. doi: 10.1083/jcb.17.1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronson C. W., Lyttleton P., Robertson J. G. C(4)-dicarboxylate transport mutants of Rhizobium trifolii form ineffective nodules on Trifolium repens. Proc Natl Acad Sci U S A. 1981 Jul;78(7):4284–4288. doi: 10.1073/pnas.78.7.4284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHWINGHAMER E. A. Studies on induced variation in the rhizobia. I. Defined media and nodulation test techniques. Appl Microbiol. 1960 Nov;8:349–352. doi: 10.1128/am.8.6.349-352.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spurr A. R. A low-viscosity epoxy resin embedding medium for electron microscopy. J Ultrastruct Res. 1969 Jan;26(1):31–43. doi: 10.1016/s0022-5320(69)90033-1. [DOI] [PubMed] [Google Scholar]