Abstract
Nonessential metal ions such as cadmium are most likely transported across plant membranes via transporters for essential cations. To identify possible pathways for Cd2+ transport we tested putative plant cation transporters for Cd2+ uptake activity by expressing cDNAs in Saccharomyces cerevisiae and found that expression of one clone, LCT1, renders the growth of yeast more sensitive to cadmium. Ion flux assays showed that Cd2+ sensitivity is correlated with an increase in Cd2+ uptake. LCT1-dependent Cd2+ uptake is saturable, lies in the high-affinity range (apparent KM for Cd2+ = 33 μM) and is sensitive to block by La3+ and Ca2+. Growth assays demonstrated a sensitivity of LCT1-expressing yeast cells to extracellular millimolar Ca2+ concentrations. LCT1-dependent increase in Ca2+ uptake correlated with the observed phenotype. Furthermore, LCT1 complements a yeast disruption mutant in the MID1 gene, a non-LCT1-homologous yeast gene encoding a membrane Ca2+ influx system required for recovery from the mating response. We conclude that LCT1 mediates the uptake of Ca2+ and Cd2+ in yeast and may therefore represent a first plant cDNA encoding a plant Ca2+ uptake or an organellar Ca2+ transport pathway in plants and may contribute to transport of the toxic metal Cd2+ across plant membranes.
Calcium is an important nutrient for plant growth and Ca2+ influx and Ca2+ release from organelles play important roles in many plant signaling cascades (1, 2). However, plant cDNAs that mediate calcium transport into the cytosol of plant cells have not yet been identified even though in recent years molecular approaches, in particular the complementation of yeast mutants, have allowed the identification of a number of plant transporters, for nutrients including sucrose (3), potassium (4–6), sulfate (7), phosphate (8–10), iron (11), and copper (12).
Cation transporters offer potential transport pathways for phytotoxic metals. For example, certain potassium channels and transporters are permeable to Na+ (13–15). Nonessential heavy metals such as cadmium are also most likely taken up via plant nutrient transporters or channels that are not completely selective. In animals voltage-gated Ca2+ channels are implicated in Cd2+ uptake (16). Voltage-dependent Ca2+ influx activities have been reported from plant plasma membranes (17–20). No plant transporter has yet been shown to mediate Cd2+ uptake into the cell. Vacuolar transporters have been cloned recently from yeast that mediate uptake of Cd-phytochelatin complexes into vacuoles (21–23) and homologous genes have been identified in Arabidopsis (24).
Understanding the mechanism of heavy metal transport across plant membranes has been proposed to aid in engineering plants with enhanced or decreased uptake (25). The uptake of heavy metal ions, including Cd2+, by agricultural plants is a major cause for the accumulation of these toxic cations in the human body (26, 27). On the other hand, plants that hyperaccumulate heavy metals might be useful for phytoremediation, i.e., the removal of toxic materials from soils and water (25, 28, 29). Physiological studies suggest that an increased metal uptake activity could be among several essential components required for metal hyperaccumulation (30). Therefore, genes encoding proteins that are involved in transport represent promising targets for multigene engineering of plant heavy metal accumulation.
We used the expression of plant cDNAs in Saccharomyces cerevisiae to test putative and known plant cation transporters and channels for their Cd2+ uptake capacity. Here we report the characterization of the recently identified wheat cDNA LCT1 that was shown to induce low-affinity Na+ and Rb+ uptake in yeast (31), as a protein mediating the uptake of Ca2+ and Cd2+ into yeast cells.
MATERIALS AND METHODS
Yeast Culture, Expression of Plant cDNAs in S. cerevisiae and Growth Assays.
INVSc1 cells (Invitrogen) were transformed with wheat cDNAs inserted into pYES2 (6) by using standard procedures and grown on yeast nitrogen base minimal medium lacking uracil. All growth assays with transformed yeast were carried out by using arginine-phosphate medium (32) containing 1 mM K+, 1% sucrose, 1% galactose. For plate assays 0.8% ultra-pure agarose (GIBCO) was added. Cells were grown overnight and either streaked out on plates or diluted into 4 ml of fresh liquid medium containing different CdCl2 or CaCl2 concentrations. Cells to be used in uptake experiments were also grown in arginine-phosphate medium.
Uptake Assays.
We found that the INVSc1 yeast line (Invitrogen) generates larger LCT1-dependent metal uptake rates than the gal− CY162 strain (4, 31). Therefore INVSc1 cells were used for uptake assays here. The isotopes 109Cd2+ and 45Ca2+ were used for uptake assays. Uptake rates were always measured in parallel for freshly transformed LCT1-expressing cells and control cells harboring the empty pYES2 plasmid. Cells were grown to an OD600 of ≈0.2, washed with H2O and resuspended in uptake solution (10 mM Hepes⋅Tris, pH 6.0/100 μM MgCl2/1% sucrose/1% galactose) to an OD600 of 0.5. After a 5 min preincubation at 30°C, uptake was started by adding either CaCl2 or CdCl2 containing 0.2–2 μCi (1 Ci = 37 GBq) of the respective isotope to the cells. Other metals used in competition experiments and inhibitors were added to the uptake assay at the beginning of the preincubation period. Total assay volume was 3.5 ml. Aliquots were taken in intervals of 2–6 min, harvested on nylon membranes (0.8 μm) and washed twice with 5 ml of 100 mM CaCl2. Radioactivity on the membranes was counted in the presence of liquid scintillation mixture (Ecoscint). LCT1-mediated Cd2+ uptake was also confirmed in pilot atomic absorption spectroscopy measurements (data not shown).
Disruption of the MID1 Locus.
The selectable marker LEU2 was inserted into the coding sequence of the MID1 gene in yeast strain C699–5 (MATa ade2 can1 his3 leu2 trp1 ura3 bar1:HisG) as described in the following. A 1,979-bp fragment of the MID1 gene was amplified from C699–5 genomic DNA by PCR using the following primers 5′-CGACCGGCTGACGTACCGT and 5′-ACTGCTAACGCCGAAGAACA. This product, containing the entire MID1 coding sequence, was cloned into the TA cloning site of pCRII (Invitrogen) to generate plasmid pMID1. The yeast LEU2 gene was obtained as a 1,732-bp fragment cloned into the EcoRV site of pBluescript. A 2,178-bp PvuII fragment containing the yeast LEU2 gene was isolated and cloned into the unique NdeI site of pMID1 to make pMID1-LEU2. The yeast strain C699–5 was transformed with a 5,864-bp BglI fragment of pMID1-LEU2 containing the interrupted MID1 gene. Transformants were selected on media lacking leucine and tested for reduced viability in the presence of α-factor by using the methylene blue liquid assay (33). Disruptions in MID1 were confirmed by using PCR and one strain (C699–5Δmid1) was selected for further experiments.
Complementation of the mid1 Mutant Phenotype.
Yeast strains C699–5 and C699–5Δmid1 were each transformed with either the pYES2 expression vector or pYES2 containing LCT1. Freshly grown colonies were picked from plates without glucose containing 2% galactose and 2% raffinose. For C699–5 the media lacked uracil and for C699–5Δmid1 the media lacked uracil and leucine. The cells were suspended in microtiter wells containing 100 μl of media [yeast nitrogen base (Difco)/2% galactose/2% raffinose/0.005% methylene blue/0.8 g liter−1 of drop-out mixture without uracil (Bio101) with and without 3 μM of the yeast peptide pheromone α-factor (Nova Biochem)]. The cells were incubated at 30°C for 3 hr, and viability was determined microscopically (33).
RESULTS
LCT1 Expression Leads to an Increase in Cd2+ Uptake.
Because Cd2+ is a nonessential element for plants (34), it is likely that plant cells do not express specific Cd2+ transporters. Certain metal uptake transporters in plants are relatively nonselective, such that both metal nutrients and toxic metals are taken up (15, 18). We therefore conducted a secondary screen among K+ uptake complementing cDNAs for those that might be involved in the transport of divalent heavy metal cations. Wheat cDNAs were expressed in the S. cerevisiae wild-type strain INVSc1 and the effects of media containing different Cd2+ concentrations on yeast growth were determined. Yeast expressing known transporters including KAT1 and HKT1 (4, 6) did not cause shifts in the Cd2+ sensitivity of yeast (data not shown). However, LCT1 (31) expression produced a dramatic increase in the Cd2+ sensitivity of yeast growth on plates. LCT1 expressing cells (YLCT1) did not grow in an arginine-phosphate medium containing 50 μM Cd2+, whereas control cells carrying the empty pYES2 plasmid were able to grow in the presence of 50 μM Cd2+ on plates (Fig. 1).
Figure 1.
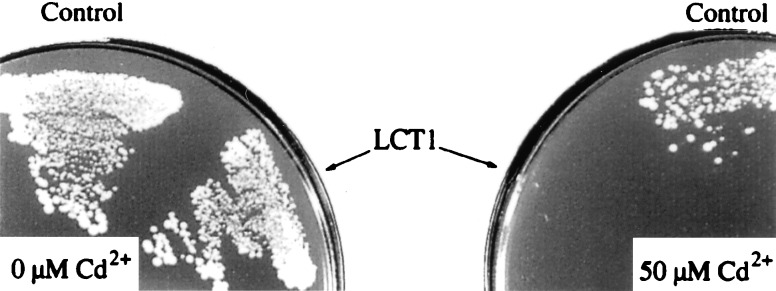
Effect of LCT1 expression on cadmium sensitivity of yeast. INVSc1 cells transformed with either the empty pYES2 plasmid (Control) or LCT1 in pYES2 were grown on arginine-phosphate medium without Cd2+ (Left) or with 50 μM Cd2+ (Right).
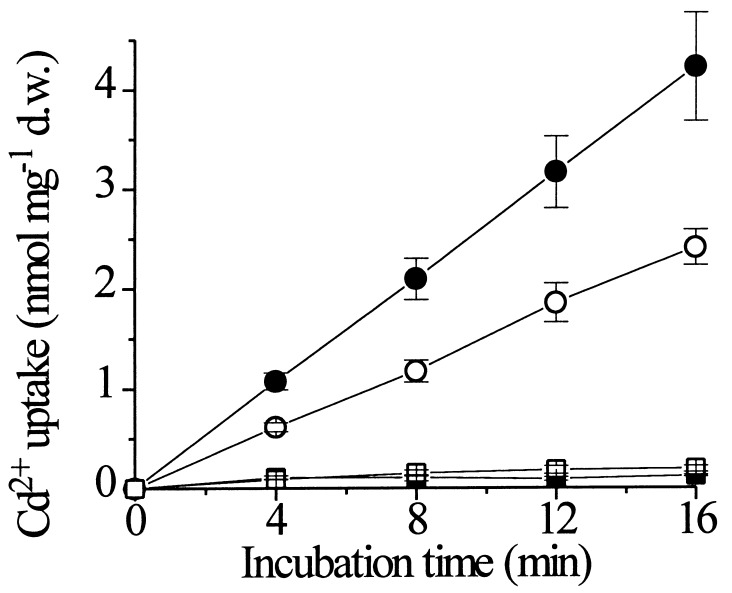
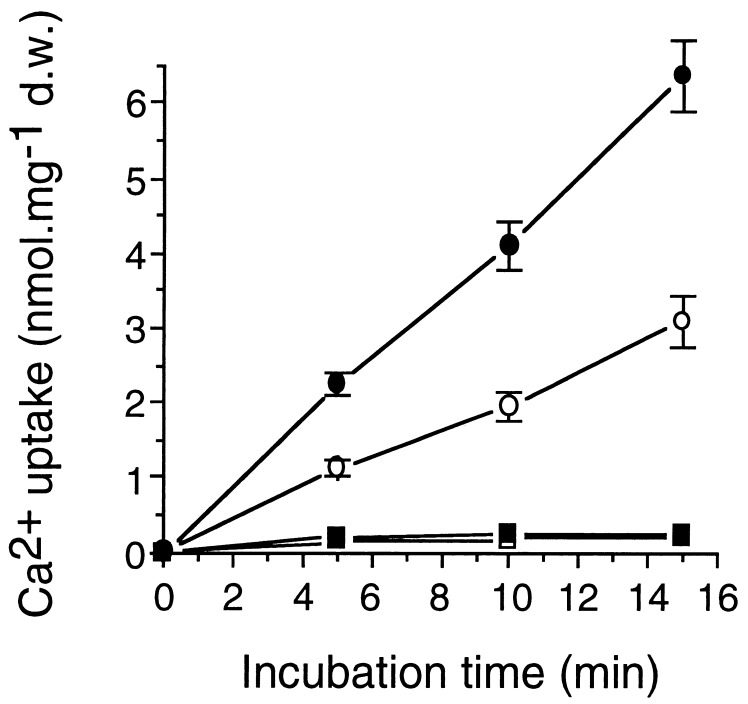
The initial characterization of LCT1 had shown that it encodes a protein with 6–8 putative membrane-spanning domains and mediates Na+ and low rates of Rb+ uptake and perhaps also Ca2+ transport in yeast (31). We pursued uptake assays for Cd2+ by using 109Cd2+ to determine whether the observed effect of Cd2+ on the growth of LCT1-expressing cells is accompanied by an increased transport rate for Cd2+. The expression of LCT1 produced an increase in Cd2+ uptake by yeast cells of ≈75% at an external concentration of 30 μM (Fig. 2, •). To determine whether cell wall binding or uptake accounted for the Cd2+ accumulation, control experiments were carried out at 0°C demonstrating that the measured amounts of Cd2+ were transported into the cells dependent on protein activity (Fig. 2, ■, □) and not simply by temperature-independent adsorption to cell walls (34). We therefore conclude, that the observed increase in Cd2+ sensitivity upon expression of LCT1 may be caused by elevated Cd2+ uptake into the yeast cells. LCT1-mediated uptake was linear for at least 20 min at 30°C.
Figure 2.
Effect of LCT1 expression on cadmium uptake in yeast. INVSc1 cells, transformed with LCT1 in pYES2 (■, •) and with the empty control pYES2 plasmid (□, ○) were grown in arginine-phosphate medium to an OD of ≈0.2 and assayed for cadmium uptake with 109Cd. Cells were incubated in uptake solution, containing 30 μM Cd2+, at 30°C (•, ○) or 0°C (■, □). Aliquots were taken at different time points and radioactivity was measured. Bars = SE; n = 8 for each data point.
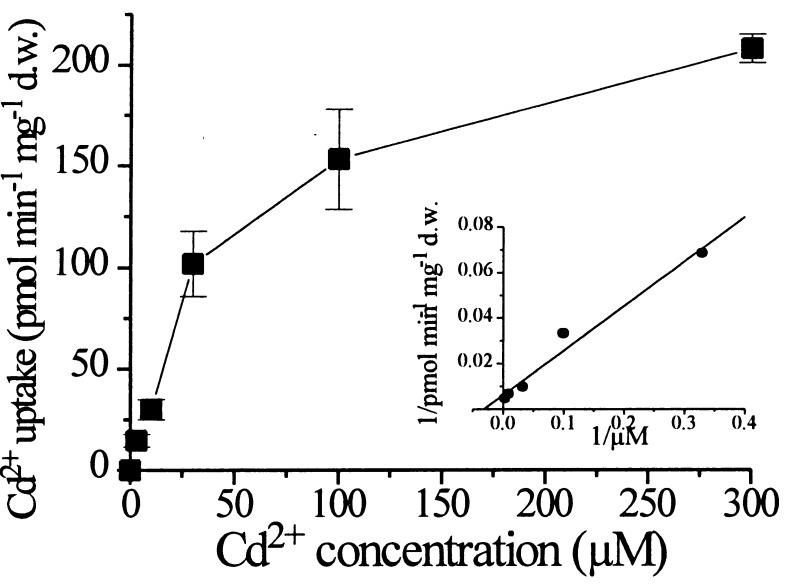
The affinity of LCT1 for Cd2+ was studied by measuring the difference in Cd2+ uptake between control cells and YLCT1 at different external Cd2+ concentrations (Fig. 3). LCT1-mediated cadmium uptake could be described by Michaelis-Menten kinetics with an apparent KM of ≈33 μM. Thus, expression of LCT1 in yeast induced an increase in Cd2+ uptake that showed saturation characteristics and an apparent affinity in the high-affinity uptake range (Fig. 3). LCT1-dependent Cd2+ uptake displayed a mild pH dependence. Uptake rates were highest at a pH of 6 and ≈30% higher at pH 6 than at pH 5 and ≈10% higher at pH 6 than at pH 7 (n = 3, data not shown).
Figure 3.
Concentration-dependence of LCT1-dependent uptake. Cd2+ uptake rates of INVSc1 control cells (carrying the empty pYES2 plasmid) were subtracted from Cd2+ uptake rates of LCT1-expressing cells to determine the LCT1-dependent Cd2+ uptake rate at different Cd2+ concentrations. Bars = SE; n = 3. The inset shows a Lineweaver–Burke plot of the uptake data. KM = 32.9 μM.
LCT1-Mediated Ca2+ Uptake.
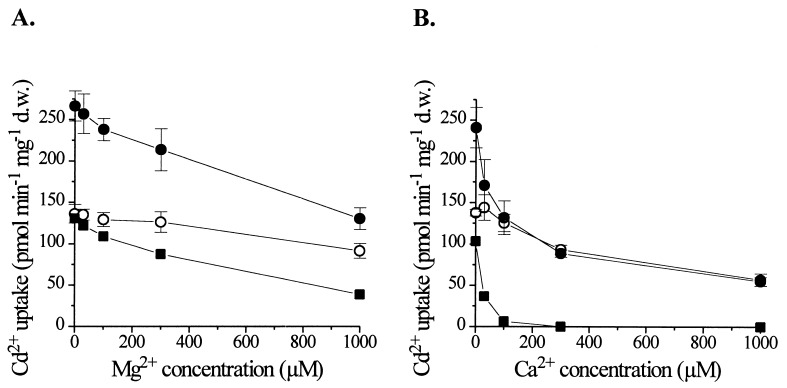
Studies in animal cells have led to the hypothesis that Cd2+ uptake is, at least in part, mediated by Ca2+ transporters (16). In addition, previous work on LCT1 had indicated a possible Ca2+ transport activity (31). We therefore tested whether LCT1 also mediates uptake of the physiological metal Ca2+. We first analyzed the competition of Mg2+ and Ca2+ ions with LCT1-dependent Cd2+ uptake (Fig. 4 A and B). The addition of Mg2+ to the uptake solution containing 30 μM Cd2+ mildly inhibited the LCT1-mediated accumulation of Cd2+ at concentrations exceeding 100 μM (Fig. 4A, ■). However, Ca2+ ions interfered strongly with the uptake of Cd2+. At concentrations of Ca2+ over 100 μM, LCT1-dependent Cd2+ uptake was strongly blocked (Fig. 4B, ■). The calculated values for half-maximal inhibition of LCT1-mediated Cd2+ uptake were 600 μM for Mg2+ and 25 μM for Ca2+. The native background Cd2+ transport activity in yeast was also affected by Ca2+, but in a different fashion. Native Cd2+ uptake was less sensitive to block by Ca2+ than the LCT1-dependent Cd2+ uptake. Half-maximal inhibition in controls occurred at a Ca2+ concentration of ≈700 μM (Fig. 4B, ○). Furthermore, LCT1-dependent Cd2+ uptake was sensitive to the channel blocker La3+. 10 μM La3+ in the uptake assay almost completely abolished the effect of LCT1 expression on Cd2+ accumulation (n = 2, data not shown). The addition of 3 μM La3+ produced an inhibition by ≈70% (n = 2). The LCT1-induced Cd2+ uptake was more sensitive to block than the native Cd2+ uptake in yeast cells that was inhibited by ≈40% and 60% at 3 μM and 10 μM La3+, respectively (n = 2).
Figure 4.
Competition of Ca2+ and Mg2+ with LCT1-dependent Cd2+ uptake. Cd2+ uptake rates were determined for INVSc1 controls (○) and LCT1-expressing cells (•) in the presence of different Mg2+ (A) and Ca2+ (B) concentrations. LCT1-dependent Cd2+ uptake (■) was determined by subtracting the rates of control cells from the rates of LCT1-expressing cells. Bars = SE; n = 4.
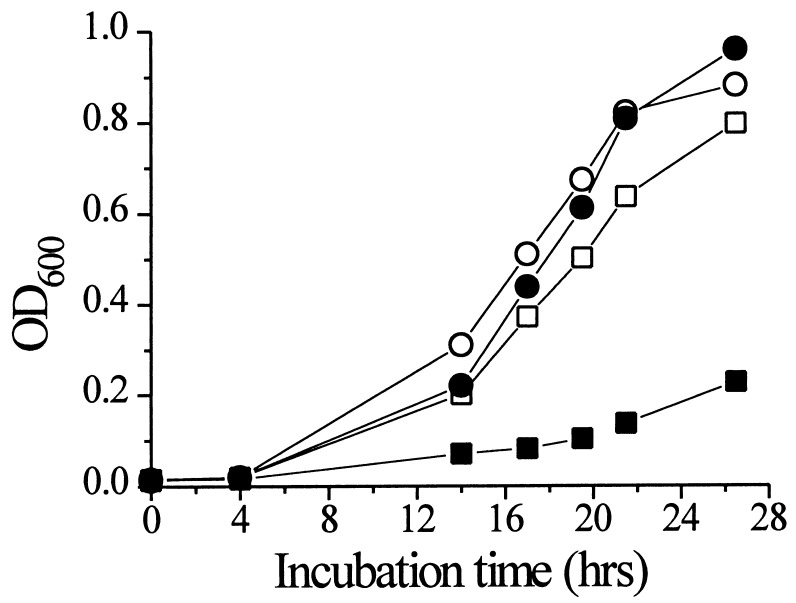
Because the Ca2+ competition data and the block by Ca2+ and La3+ indicated a possible Ca2+ transport activity of LCT1, we investigated the effects of different Ca2+ concentrations in the medium on yeast growth. Excess Ca2+ accumulation in yeast is toxic (36). Thus, the unregulated overexpression of a Ca2+-transporting protein should render the growth of yeast cells sensitive to elevated external Ca2+ concentrations. At 10 μM Ca2+ in argininine-phosphate medium no significant differences were observed in growth of control and LCT1-expressing yeast cells (Fig. 5, ○, •). However, at 3 mM Ca2+ growth of LCT1-expressing cells was severely inhibited (Fig. 5, ■) whereas control cells tolerated this concentration with only a minor reduction in growth rate (Fig. 5, □). Half-maximal inhibition of growth occurred at a Ca2+ concentration of 1.7 mM for LCT1-expressing cells. This finding provides an indication for LCT1-mediated enhancement of Ca2+ uptake. This hypothesis was directly tested by measuring Ca2+ uptake rates. Uptake assays using 45Ca2+ demonstrated an LCT1-dependent increase in Ca2+ uptake (Fig. 6). At an external Ca2+ concentration of 100 μM YLCT1 took up approximately twice as much Ca2+ as control cells (Fig. 6, •). Uptake assays at 0°C showed that protein-dependent uptake into the cells and not adsorption to the cell wall accounted for the observed accumulation of Ca2+ (Fig. 6, ■, □). LCT1-dependent Ca2+ uptake was linear up to a concentration of at least 3 mM (n = 5, data not shown).
Figure 5.
Ca2+ sensitivity of LCT1-expressing cells. LCT1-expressing cells (■, •) and INVSc1 control cells (□, ○) were grown in arginine-phosphate medium containing either 10 μM Ca2+ (•, ○) or 3 mM Ca2+ (■, □).
Figure 6.
Effect of LCT1 expression on calcium uptake in yeast. INVSc1 cells transformed with LCT1 in pYES2 (■, •) and with the empty pYES2 control plasmid (□, ○) were grown in arginine-phosphate medium to an OD of ≈0.2 and assayed for calcium uptake with 45Ca2+. Cells were incubated in uptake solution, containing 100 μM Ca2+, at 30°C (•, ○) or 0°C (■, □). Aliquots were taken at different time points and radioactivity was measured. Bars = SE; n = 7 for each data point.
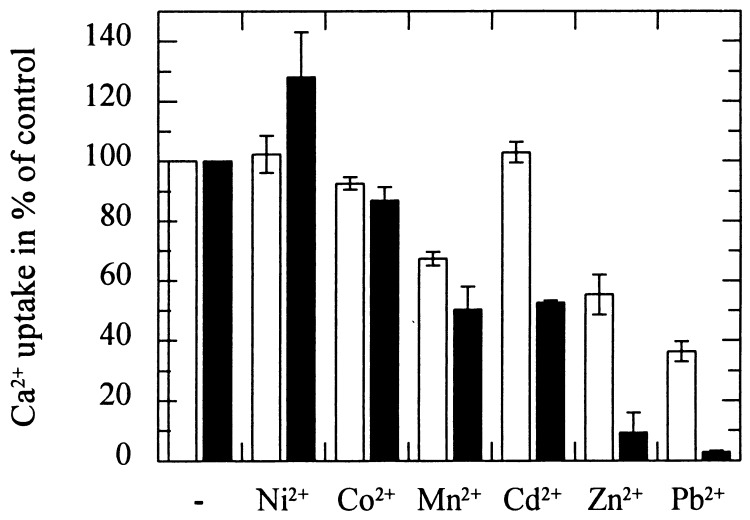
To further test whether LCT1-mediated Ca2+ uptake can be distinguished from native uptake in control cells, effects of other heavy metals on LCT1-mediated Ca2+ uptake were studied. The addition of 10 μM Pb2+ or 100 μM Zn2+ abolished LCT1-mediated Ca2+ uptake almost completely (Fig. 7, ■). Mn2+ (100 μM) and Cd2+ (100 μM) also inhibited LCT1-mediated Ca2+ uptake significantly whereas Co2+ had only a mild effect and Ni2+ addition led to a small increase in uptake. The competition of Cu2+ could not be determined because Cu2+ stimulated the native Ca2+ uptake system in yeast cells leading to a several-fold increase in Ca2+ accumulation that masked the effect of LCT1 expression (n = 3, data not shown). As found above for the Ca2+ and La3+ sensitivity of Cd2+ uptake (Fig. 4B), the native background Ca2+ uptake was less sensitive to some of the cations tested (Fig. 7, □). In particular, addition of 100 μM Zn2+ caused only a 45% reduction in Ca2+ accumulation and 10 μM Pb2+ a reduction by 64%. A concentration of 100 μM Cd2+ did not affect the wild-type uptake rate (Fig. 7).
Figure 7.
Effect of different divalent cations on LCT1-dependent Ca2+ uptake. Ca2+ uptake of LCT1-expressing cells (■) and INVSc1 control cells (□) was measured in the presence of other cations. The external Ca2+ concentration was 100 μM, the concentration of Pb2+ was 10 μM, the concentration of the other cations was 100 μM. The uptake rates are shown as % of controls that had no competing metal added to the uptake assay. Native background Ca2+ uptake (□) is compared with LCT1-dependent Ca2+ uptake (■). LCT1-dependent Ca2+ uptake was determined by subtracting the rate of control cells from the rate determined for LCT1-expressing cells. The average Ca2+ uptake rate in these experiments was 213.0 (±20.1) pmol⋅min−1⋅mg−1 d.w. for control cells and 444.0 (±26.4) pmol⋅min−1⋅mg−1 dry weight for LCT1-expressing cells. Error bars represent SE, n = 3 for each condition.
LCT1 Complements a mid1 Knock-Out.
To further test our hypothesis that LCT1 encodes a Ca2+ transporter, we analyzed its ability to complement a S. cerevisiae strain with a disruption of the MID1 gene. MID1 encodes an integral plasma membrane protein mediating Ca2+ influx required for recovery from the yeast mating response (33, 37). The C699–5 yeast strain was selected for disruption of the MID1 gene and complementation experiments. This strain is hypersensitive to α-factor due to the disruption of the BAR1 gene that encodes a protease that degrades α-factor (38). In the present experiments, 54 potential mid1 knock-out mutants were selected by growth on media without leucine. Of these, nine transformants were found to display reduced viability in the presence of α-factor as reported for mid1 mutants (33). All of these were found to contain the expected complete knock-outs in the MID1 gene.
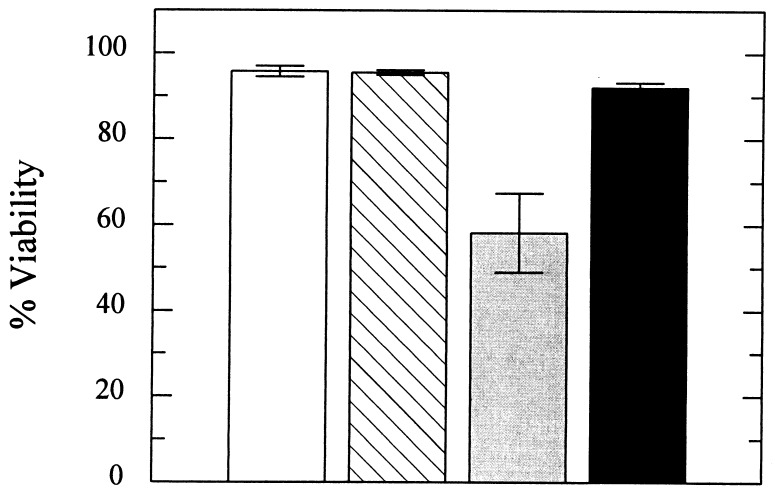
A typical morphological response to α-factor, the change into cells with one or more projections on the cell surface (“shmoos,” ref. 37), was observed for both yeast strains. The control yeast strain C699–5, containing either pYES2 or pYES2-LCT1 displayed no reduction in viability after treatment with α-factor for 3 hr (Fig. 8, open bar, striped bar). However, viability of the C699–5Δmid1 yeast containing pYES2 decreased to 58% within 3 hr (Fig. 8, ░⃞). This decrease is similar to the level of decrease in viability reported previously in mid1 mutants under the same conditions (33). Expression of LCT1 in the mid1 mutant prevented this reduced viability, only 8% of the yeast cells displayed methylene blue uptake (Fig. 8, ■). Therefore, LCT1 complemented the mid1 mutation.
Figure 8.
Expression of LCT1 in the mid1 mutant prevented cell death in response to mating pheromone compared with non-LCT1-expressing mid1 cells. Yeast viability was determined by using the methylene blue liquid method. Cells were incubated for 3 hr in the presence of 3 μM α-factor. Four hundred yeast cells were scored for uptake of methylene blue by microscopic observation for each strain-plasmid combination [□, C699–5 (pYES2); ▧, C699–5 (LCT1); ░⃞, C699–5Δmid1 (pYES2); ■, C699–5Δmid1 (LCT1)] in this experiment and two independent transformants were tested for each with the same result. Bars = SD.
DISCUSSION
The complementation of a mid1 knock-out mutant by LCT1 together with the effects of LCT1-expression on yeast growth sensitivity to Ca2+ and Cd2+ (Figs. 1 and 5) and on yeast uptake of Ca2+ and Cd2+ (Figs. 2 and 6) support the hypothesis that LCT1 encodes a transport activity mediating the uptake of Ca2+ and Cd2+ across the plasma membrane of yeast.
LCT1 Mediates Cd2+ and Ca2+ Uptake in Yeast.
We found that overexpression of the wheat cDNA LCT1 (31) dramatically increased the Cd2+ sensitivity of S. cerevisiae growth on plates. This effect is less pronounced in liquid culture, possibly due to different external conditions, such as removal of local external ion gradients in liquid culture. Cd2+ uptake assays confirmed that the observed phenotype correlated with higher Cd2+ uptake rates of LCT1-expressing cells with distinct properties to native Cd2+ uptake (Figs. 2, 3, and 4B). In contrast to a number of studies on plant ion transporters expressed in yeast, we were not able to use a control yeast mutant deficient in the respective uptake activity (3, 4, 11). This explains the considerable background uptake of controls. The rates for LCT1-dependent Cd2+ uptake at different external Cd2+ concentrations showed a component with an apparent KM of 33 μM for Cd2+.
The uptake of nonessential and phytotoxic Cd2+ ions suggests that transport of beneficial cations may be the natural function of LCT1. Schachtman et al. (31) demonstrated an increase in Rb+ uptake of LCT1-expressing cells. The low rates and the low affinity, however, led to the conclusion that LCT1 does not contribute significantly to potassium nutrition (31) and a physiological role of LCT1 therefore remained to be determined. Cd2+ and other heavy metal cations are known to interact with Ca2+ channels, Ca2+ transporters and Ca2+ binding proteins (39–41). Furthermore, studies in animal cells suggested Cd2+ uptake via voltage-gated Ca2+ channels (16). Plants have to take up calcium from the soil via the root system. Huang et al. (18) described depolarization-activated Ca2+ influx into wheat root vesicles. Several different pathways for calcium uptake are likely to exist in plants that can play diverse roles in signal transduction and Ca2+ nutrition. Hyperpolarization and elicitor-activated channels in tomato cells (42), elicitor-activated Ca2+ permeable channels in parsley cells (43), and ABA-activated nonselective Ca2+ permeable channels in guard cells (44) have been characterized. We note that in plant cells, LCT1 may be targeted to organellar membranes or to the plasma membrane. In either case, to our knowledge no plant cDNA has yet been shown to mediate Ca2+ influx or organellar Ca2+ release and LCT1 is a first putative candidate contributing to one of these functions.
The differential effect of Mg2+ and Ca2+ on LCT1-dependent Cd2+ uptake (Fig. 4 A and B) and the La3+ sensitivity indicated that LCT1 might function as a Ca2+ transport system. Growth assays and uptake experiments demonstrated that LCT1 expression induced a significant protein-dependent increase in Ca2+ uptake of yeast cells. A KM for Ca2+ could not be determined because LCT1-dependent Ca2+ influx does not show saturation up to a concentration of at least 3 mM. The growth inhibition of LCT1 expressing cells at different Ca2+ concentrations indicated an apparent KM in the millimolar range. This seems to contradict the high-affinity binding of Ca2+ indicated by the strong block of Cd2+ uptake (Fig. 4B.). However, it has been often found that Ca2+ channels possess multiple ion binding sites and show a high affinity binding of Ca2+ but a low affinity for Ca2+ conductance (45).
We propose from these data that LCT1 may function as a Ca2+ transport system, possibly contributing to either Ca2+ influx across the plasma membrane or Ca2+ transport across organelle membranes in plants. LCT1-mediated cation uptake is nonselective allowing permeation of both Cd2+ (Fig. 2) and Na+ (31). Low-affinity Na+ uptake mediated by LCT1 has led to the suggestion that LCT1 could provide one of the pathways for Na+ transport into the cytosol of plant cells (31). LCT1-mediated Ca2+ and Na+ transport are consistent with the observation in plants that Ca2+ reduces transport of Na+ (46). Note that intracellular Ca2+ regulation, as a second messenger, also contributes to Ca2+ regulation of Na+ transport.
Experiments testing the effect of other divalent cations on LCT1-induced Ca2+ uptake showed the expected inhibition by Cd2+. Zn2+, and Pb2+ blocked Ca2+ uptake even more efficiently. Zn2+ was shown by Schachtman et al. (31) to be a potent blocker of Rb+ uptake that is not transported by LCT1. Further uptake studies are necessary to test whether Pb2+ is a transported substrate for LCT1. The low selectivity of LCT1 indicates that LCT1 may mediate the transport of additional cations.
LCT1 Complements a mid1 Knock-Out.
The MID1-mediated influx of Ca2+ in yeast after mating response is a regulated process (33, 37). However, the finding that elevated Ca2+ in the medium prevents cell death in Δmid1 mutants (33) led us to hypothesize that heterologously expressed transporters, such as LCT1 that allow Ca2+ influx in yeast, but are not expected to respond to the mating signal transduction pathway might complement this mutant. We decided to construct a mid1-knock-out strain to further test the hypothesis that LCT1 functions as a Ca2+ influx system in yeast. Analysis of the mating factor response of control cells, the Δmid1 knock-out strain and the Δmid1 knock-out strain expressing LCT1 clearly demonstrated that LCT1 complements the Δmid1 disruption. These data provide additional strong evidence that LCT1 represents a Ca2+ transporter. In addition, the results of these experiments indicate that the utilization of mid1 mutants could provide a means to test Ca2+ uptake by other transporters from plants or animals.
Does LCT1 Encode the Transporter Itself?
The LCT1-dependent increases in Ca2+ and Cd2+ uptake rates could theoretically be explained as an activation of yeast uptake systems by LCT1. However, there are no homologous sequences in the completely sequenced S. cerevisiae genome (47) and the amino acid sequence of LCT1 displays the hydrophobic domains reminiscent of a membrane protein (31). Furthermore, LCT1-dependent Ca2+ and Cd2+ uptake displayed some characteristics different from the native background uptake. LCT1 induced a Cd2+ influx component that is significantly more sensitive to external Ca2+ and La3+ than Cd2+ influx measured in control cells (Fig. 4B). Cd2+, Mn2+, Zn2+, and Pb2+ inhibited the LCT1-dependent Ca2+ uptake more strongly than the wild-type Ca2+ uptake (Fig. 7). In addition, Δmid1 complementation demonstrated that LCT1 can replace a yeast component of a plasma membrane Ca2+ influx system.
This evidence supports the hypothesis that LCT1 encodes a membrane protein permeable to Ca2+ and Cd2+. The activation of an endogenous transport activity by LCT1, however, cannot be entirely ruled out. Whether the LCT1 protein is targeted to the plasma membrane or organellar membranes in plants will be addressed in future studies. Although Ca2+ influx into cells does not require active transport, the question whether LCT1 encodes a Ca2+-permeable channel or a carrier mechanism remains to be addressed.
A Role for LCT1 in Plant Heavy Metal Transport?
To our knowledge, at present no plant cDNA has been shown to mediate the influx of Cd2+ or other nonessential, phytotoxic heavy metals. Different pathways for the entry of cadmium into the cytosol should exist. For instance, iron transport mediated by IRT1 is sensitive to Cd2+ block (11) and Cd2+ uptake remains to be analyzed. The presented effect of LCT1-expression on Cd2+ uptake in yeast suggests that LCT1 might contribute to cadmium transport in plants. Taking the Ca2+ sensitivity of LCT1-dependent Cd2+ uptake into account, however, the Cd2+ permeability of LCT1 is probably not relevant in soils with high Ca2+ concentrations unless local Ca2+ depletion occurs.
Heavy metal uptake is one of the essential components of heavy metal hyperaccumulation. It is, however, not clear whether uptake rates are a limiting factor. Studies on the hyperaccumulating plant Thlaspi caerulescens and the nonhyperaccumulating plant Thlaspi arvense show higher Zn2+ uptake rates for the hyperaccumulating species (30). Therefore, modification of uptake transporters may represent a target for multigenic enhancement of heavy metal hyperaccumulation.
In conclusion, we have shown that expression of the wheat cDNA LCT1 in S. cerevisiae leads to an increase in Ca2+ and Cd2+ uptake with a high affinity for Cd2+. Physiologically LCT1 may function in plant cell Ca2+ influx or organellar Ca2+ transport. The membrane targeting and tissue localization of LCT1 in plants remains to be determined. Ca2+ transport mediated by LCT1 was demonstrated by measuring the Ca2+ sensitivity and Ca2+ uptake of LCT1-expressing yeast cells, by characterizing competition of cations with Cd2+ and Ca2+ and by complementing the knock-out of MID1, a yeast plasma membrane Ca2+-influx system. To our knowledge, this is the first report of a plant cDNA that mediates both Ca2+ and Cd2+ transport into the cytosol of cells.
Acknowledgments
We thank Drs. Beverly Errede and Scott Emr for the yeast strain C699-5 and the LEU2 gene, Drs. Nigel Crawford and Gethyn Allen for helpful comments on the manuscript: This work was supported by U.S. Department of Energy Grant DE-FG07-96ER20253 to J.I.S., National Science Foundation Grant MCB-9506191 to J.I.S., and a DAAD-North Atlantic Treaty Organization postdoctoral fellowship to S.C.
References
- 1. Bush D S. Annu Rev Plant Physiol Plant Mol Biol. 1995;46:95–122. [Google Scholar]
- 2.Webb A A R, McAinsh M R, Taylor J E, Hetherington A M. Adv Bot Res. 1996;22:45–96. [Google Scholar]
- 3.Riesmeier J W, Willmitzer L, Frommer W B. EMBO J. 1992;11:4705–4713. doi: 10.1002/j.1460-2075.1992.tb05575.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Anderson J A, Huprikar S S, Kochian L V, Lucas W J, Gaber R F. Proc Natl Acad Sci USA. 1992;89:3736–3740. doi: 10.1073/pnas.89.9.3736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sentenac H, Bonneaud N, Minet M, Lacroute F, Salmon J, Gaymard F, Grignon C. Science. 1992;256:663–665. doi: 10.1126/science.1585180. [DOI] [PubMed] [Google Scholar]
- 6.Schachtman D P, Schroeder J I. Nature (London) 1994;370:655–658. doi: 10.1038/370655a0. [DOI] [PubMed] [Google Scholar]
- 7.Smith F W, Ealing P M, Hawkesford M J, Clarkson D T. Proc Natl Acad Sci USA. 1995;92:9373–9378. doi: 10.1073/pnas.92.20.9373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Muchhal U S, Pardo J M, Raghothama K G. Proc Natl Acad Sci USA. 1996;93:10519–10523. doi: 10.1073/pnas.93.19.10519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Smith F W, Ealing P M, Dong B, Delhaize E. Plant J. 1997;11:83–92. doi: 10.1046/j.1365-313x.1997.11010083.x. [DOI] [PubMed] [Google Scholar]
- 10.Leggewie G, Willmitzer L, Riesmeier J W. Plant Cell. 1997;9:381–392. doi: 10.1105/tpc.9.3.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eide D, Broderius M, Fett J, Guerinot M L. Proc Natl Acad Sci USA. 1996;93:5624–5628. doi: 10.1073/pnas.93.11.5624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kampfenkel K, Kushnir S, Babiychuk E, Inze D, Van Montagu M. J Biol Chem. 1995;270:28479–28486. doi: 10.1074/jbc.270.47.28479. [DOI] [PubMed] [Google Scholar]
- 13.Stoeckel H, Takeda K. Proc R Soc London Ser B. 1989;237:213–231. [Google Scholar]
- 14.Schachtman D P, Tyerman S D, Terry B R. Plant Physiol. 1991;97:598–605. doi: 10.1104/pp.97.2.598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rubio F, Gassmann W, Schroeder J I. Science. 1995;270:1660–1663. doi: 10.1126/science.270.5242.1660. [DOI] [PubMed] [Google Scholar]
- 16.Hinkle P M, Shanshala E D, Nelson E J. J Biol Chem. 1992;267:25553–25559. [PubMed] [Google Scholar]
- 17.Marshall J, Corzo A, Leigh R A, Sanders D. Plant J. 1994;5:683–694. [Google Scholar]
- 18.Huang J W, Grunes D L, Kochian L V. Proc Natl Acad Sci USA. 1994;91:3473–3477. doi: 10.1073/pnas.91.8.3473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pineros M, Tester M. Planta. 1995;195:478–488. [Google Scholar]
- 20.Thuleau P, Ward J M, Ranjeva R, Schroeder J I. EMBO J. 1994;13:2970–2975. doi: 10.1002/j.1460-2075.1994.tb06595.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ortiz D F, Ruscitti T, McCue K F, Ow D W. J Biol Chem. 1995;270:4721–4728. doi: 10.1074/jbc.270.9.4721. [DOI] [PubMed] [Google Scholar]
- 22.Tommasini R, Evers R, Vogt E, Mornet C, Zaman G J, Schinkel A H, Borst P, Martinoia E. Proc Natl Acad Sci USA. 1996;93:6743–6748. doi: 10.1073/pnas.93.13.6743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li Z S, Szczypka M, Lu Y P, Thiele D J, Rea P A. J Biol Chem. 1996;271:6509–6517. doi: 10.1074/jbc.271.11.6509. [DOI] [PubMed] [Google Scholar]
- 24.Lu Y P, Li Z S, Rea P A. Proc Natl Acad Sci USA. 1997;94:8243–8248. doi: 10.1073/pnas.94.15.8243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Raskin I. Proc Natl Acad Sci USA. 1996;93:3164–3166. doi: 10.1073/pnas.93.8.3164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wagner G J. Adv Agron. 1994;51:173–212. [Google Scholar]
- 27.Van Bruwaene R, Kirchmann R, Impens R. Experientia. 1984;40:43–52. doi: 10.1007/BF01959101. [DOI] [PubMed] [Google Scholar]
- 28.Moffat A S. Science. 1995;269:302–303. doi: 10.1126/science.269.5222.302. [DOI] [PubMed] [Google Scholar]
- 29.Cunningham S D, Ow D W. Plant Physiol. 1996;110:715–719. doi: 10.1104/pp.110.3.715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lasat M M, Baker A J M, Kochian L V. Plant Physiol. 1996;112:1715–1722. doi: 10.1104/pp.112.4.1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schachtman D P, Kumar R, Schroeder J I, Marsh E L. Proc Natl Acad Sci USA. 1997;94:11079–11084. doi: 10.1073/pnas.94.20.11079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rodriguez-Navarro A, Ramos J. J Bacteriol. 1984;159:940–945. doi: 10.1128/jb.159.3.940-945.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Iida H, Nakamura H, Ono T, Okumura M S, Anraku Y. Mol Cell Biol. 1994;14:8259–8271. doi: 10.1128/mcb.14.12.8259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Marschner H. Mineral Nutrition of Higher Plants. 2nd Ed. New York: Academic; 1995. [Google Scholar]
- 35.Kochian L. Annu Rev Plant Physiol Plant Mol Biol. 1995;46:237–260. [Google Scholar]
- 36.Cunningham K W, Fink G R. J Exp Biol. 1994;196:157–166. doi: 10.1242/jeb.196.1.157. [DOI] [PubMed] [Google Scholar]
- 37.Iida H, Yagawa Y, Anraku Y. J Biol Chem. 1990;265:13391–13399. [PubMed] [Google Scholar]
- 38.Chan R K, Otte C A. Mol Cell Biol. 1982;2:11–20. doi: 10.1128/mcb.2.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Reuter H. Nature (London) 1983;301:569–574. doi: 10.1038/301569a0. [DOI] [PubMed] [Google Scholar]
- 40.Hille B. Ionic Channels of Excitable Membranes. 2nd Ed. Sunderland, MA: Sinauer; 1992. [Google Scholar]
- 41.Benters J, Flogel U, Schäfer T, Leibfritz D, Hechtenberg S, Beyersmann D. Biochem J. 1997;322:793–799. doi: 10.1042/bj3220793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gelli A, Blumwald E. J Membr Biol. 1997;155:35–45. doi: 10.1007/s002329900156. [DOI] [PubMed] [Google Scholar]
- 43.Zimmermann S, Nürnberger T, Frachisse J M, Wirtz W, Guern J, Hedrich R, Scheel D. Proc Natl Acad Sci USA. 1997;94:2751–2755. doi: 10.1073/pnas.94.6.2751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schroeder J I, Hagiwara S. Proc Natl Acad Sci USA. 1990;87:9305–9309. doi: 10.1073/pnas.87.23.9305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Almers W, McCleskey E W. J Physiol (London) 1984;353:585–608. doi: 10.1113/jphysiol.1984.sp015352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.LaHaye P A, Epstein E. Science. 1969;166:395–396. doi: 10.1126/science.166.3903.395. [DOI] [PubMed] [Google Scholar]
- 47.Mewes, H. W., Albermann, K., Bahr, M., Frishman, D., Gleissner, A., Hani, J., Heumann, K., Kleine, K., Maierl, A., Oliver, S. G., et al. (1997) Nature (London)387, Suppl., 7–65. [DOI] [PubMed]