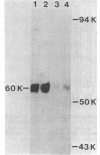
Abstract
Heating competent Azotobacter vinelandii at 37 or 42 degrees C resulted in a total loss of competence with no loss of viability. The transformation process was relatively insensitive to heating at either temperature once DNase-resistant DNA binding was nearly complete. Although competent and 42 degrees C-treated cells bound equivalent amounts of [32P]DNA in a DNase-resistant state, no donor DNA marker (nif) or radioactivity was detected in the envelope-free cell lysate of heated cells, suggesting that DNA transport across the cell envelope was a heat-sensitive event. Competence was reacquired in a 42 degrees C-treated culture after 2 h of incubation at 30 degrees C by a process which required RNA and protein syntheses. The release of a surface glycoprotein, required for competence, from cells treated at 42 degrees C occurred in an insufficient amount to account for the total loss of competence. Recovery of competence in 42 degrees C-treated cells and further transformation of competent cells were prevented by the exposure of cells to saturating amounts of transforming DNA. Further DNase-resistant DNA binding, however, still occurred, suggesting that there were two types of receptors for DNase-resistant DNA binding to competent A. vinelandii. DNase-resistant DNA binding was dependent on magnesium ions, and at least one receptor type did not discriminate against heterologous DNA.
Full text
PDF


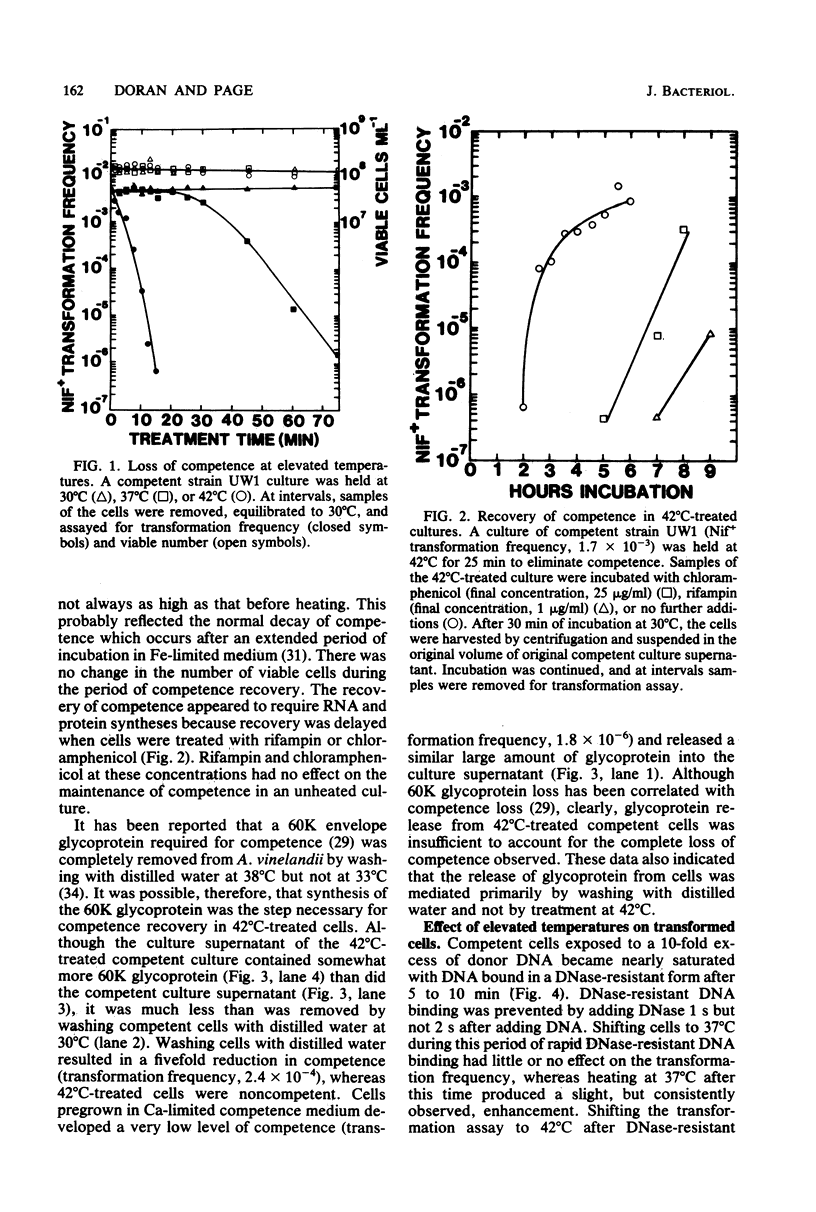
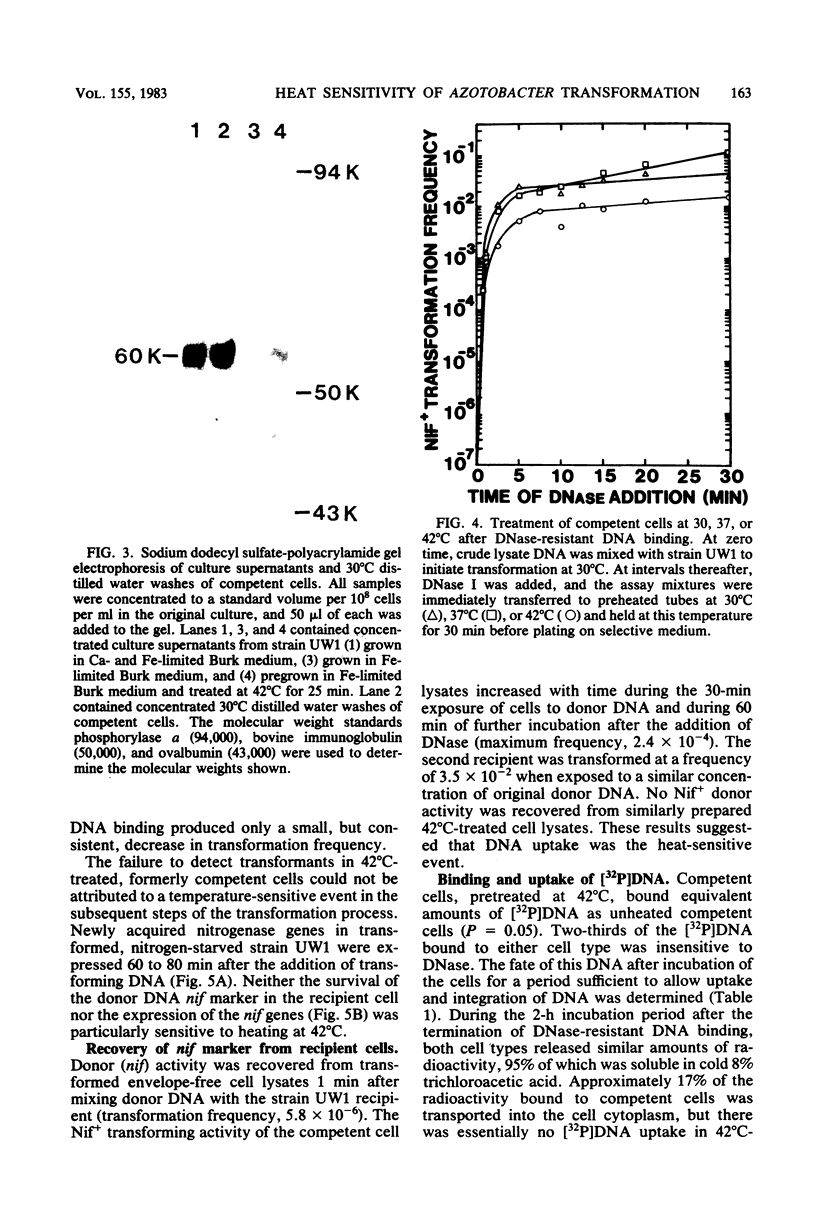
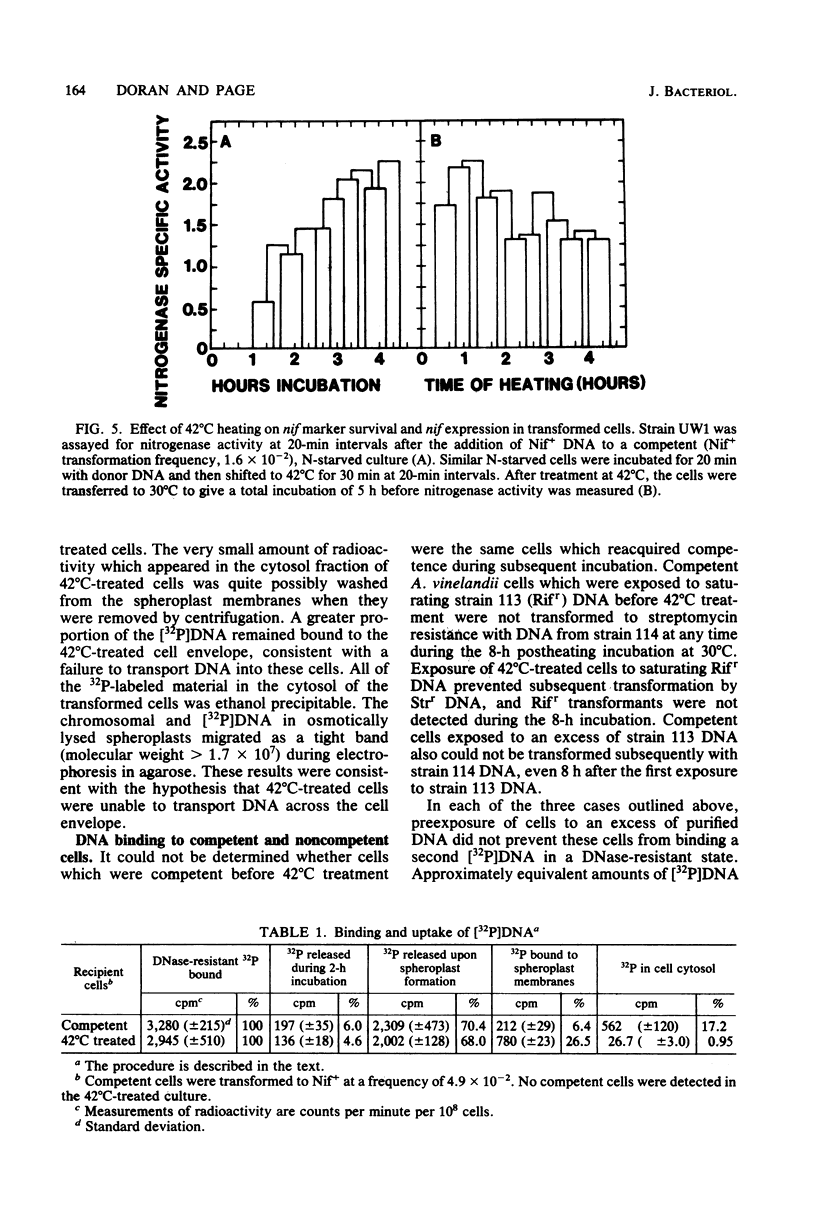




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BARNHART B. J., HERRIOTT R. M. PENETRATION OF DEOXYRIBONUCLEIC ACID INTO HEMOPHILUS INFLUENZAE. Biochim Biophys Acta. 1963 Sep 17;76:25–39. [PubMed] [Google Scholar]
- BURTON K. A study of the conditions and mechanism of the diphenylamine reaction for the colorimetric estimation of deoxyribonucleic acid. Biochem J. 1956 Feb;62(2):315–323. doi: 10.1042/bj0620315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswas G. D., Sparling P. F. Entry of double-stranded deoxyribonucleic acid during transformation of Neisseria gonorrhoeae. J Bacteriol. 1981 Jan;145(1):638–640. doi: 10.1128/jb.145.1.638-640.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson C. A., Pierson L. S., Rosen J. J., Ingraham J. L. Pseudomonas stutzeri and related species undergo natural transformation. J Bacteriol. 1983 Jan;153(1):93–99. doi: 10.1128/jb.153.1.93-99.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S. N., Chang A. C., Hsu L. Nonchromosomal antibiotic resistance in bacteria: genetic transformation of Escherichia coli by R-factor DNA. Proc Natl Acad Sci U S A. 1972 Aug;69(8):2110–2114. doi: 10.1073/pnas.69.8.2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Concino M. F., Goodgal S. H. Haemophilus influenzae polypeptides involved in deoxyribonucleic acid uptake detected by cellular surface protein iodination. J Bacteriol. 1981 Oct;148(1):220–231. doi: 10.1128/jb.148.1.220-231.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David M., Tronchet M., Dénarié J. Transformation of Azotobacter vinelandii with plasmids RP4 (IncP-1 group) and RSF1010 (IncQ group). J Bacteriol. 1981 Jun;146(3):1154–1157. doi: 10.1128/jb.146.3.1154-1157.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deddish P. A., Ravin A. W. Relation of macromolecular synthesis in streptococci to efficiency of transformation by markers of homospecific and heterospecific origin. J Bacteriol. 1974 Mar;117(3):1158–1170. doi: 10.1128/jb.117.3.1158-1170.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deich R. A., Hoyer L. C. Generation and release of DNA-binding vesicles by Haemophilus influenzae during induction and loss of competence. J Bacteriol. 1982 Nov;152(2):855–864. doi: 10.1128/jb.152.2.855-864.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deich R. A., Smith H. O. Mechanism of homospecific DNA uptake in Haemophilus influenzae transformation. Mol Gen Genet. 1980 Feb;177(3):369–374. doi: 10.1007/BF00271475. [DOI] [PubMed] [Google Scholar]
- Dougherty T. J., Asmus A., Tomasz A. Specificity of DNA uptake in genetic transformation of gonococci. Biochem Biophys Res Commun. 1979 Jan 15;86(1):97–104. doi: 10.1016/0006-291x(79)90386-3. [DOI] [PubMed] [Google Scholar]
- Fairbanks G., Steck T. L., Wallach D. F. Electrophoretic analysis of the major polypeptides of the human erythrocyte membrane. Biochemistry. 1971 Jun 22;10(13):2606–2617. doi: 10.1021/bi00789a030. [DOI] [PubMed] [Google Scholar]
- Graves J. F., Biswas G. D., Sparling P. F. Sequence-specific DNA uptake in transformation of Neisseria gonorrhoeae. J Bacteriol. 1982 Dec;152(3):1071–1077. doi: 10.1128/jb.152.3.1071-1077.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy R. W., Holsten R. D., Jackson E. K., Burns R. C. The acetylene-ethylene assay for n(2) fixation: laboratory and field evaluation. Plant Physiol. 1968 Aug;43(8):1185–1207. doi: 10.1104/pp.43.8.1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahn M. E., Maul G., Goodgal S. H. Possible mechanism for donor DNA binding and transport in Haemophilus. Proc Natl Acad Sci U S A. 1982 Oct;79(20):6370–6374. doi: 10.1073/pnas.79.20.6370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LERMAN L. S., TOLMACH L. J. Genetic transformation. I. Cellular incorporation of DNA accompanying transformation in Pneumococcus. Biochim Biophys Acta. 1957 Oct;26(1):68–82. doi: 10.1016/0006-3002(57)90055-0. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lacks S., Greenberg B. Competence for deoxyribonucleic acid uptake and deoxyribonuclease action external to cells in the genetic transformation of Diplococcus pneumoniae. J Bacteriol. 1973 Apr;114(1):152–163. doi: 10.1128/jb.114.1.152-163.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacks S., Greenberg B., Neuberger M. Role of a deoxyribonuclease in the genetic transformation of Diplococcus pneumoniae. Proc Natl Acad Sci U S A. 1974 Jun;71(6):2305–2309. doi: 10.1073/pnas.71.6.2305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- López P., Espinosa M., Piechowska M., Shugar D. Influence of bacteriophage PBS1 and phi W-14 deoxyribonucleic acids on homologous deoxyribonucleic acid uptake and transformation in competent Bacillus subtilis. J Bacteriol. 1980 Jul;143(1):50–58. doi: 10.1128/jb.143.1.50-58.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MASTER R. W. POSSIBLE SYNTHESIS OF POLYRIBONUCLEOTIDES OF KNOWN BASE-TRIPLET SEQUENCES. Nature. 1965 Apr 3;206:93–93. doi: 10.1038/206093b0. [DOI] [PubMed] [Google Scholar]
- McCarthy C., Nester E. W. Heat-sensitive step in deoxyribonucleic acid-mediated transformation of Bacillus subtilis. J Bacteriol. 1969 Jan;97(1):162–165. doi: 10.1128/jb.97.1.162-165.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald W. C. Initial temperature sensitivity of streptomycin-dependent and tryptophan transformants of Bacillus subtilis. Can J Microbiol. 1971 Jun;17(6):823–825. doi: 10.1139/m71-132. [DOI] [PubMed] [Google Scholar]
- Mulder J. A., Venema G. Transformation-deficient mutants of Bacillus subtilis impaired in competence-specific nuclease activities. J Bacteriol. 1982 Oct;152(1):166–174. doi: 10.1128/jb.152.1.166-174.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page W. J., Doran J. L. Recovery of competence in calcium-limited Azotobacter vinelandii. J Bacteriol. 1981 Apr;146(1):33–40. doi: 10.1128/jb.146.1.33-40.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page W. J., Sadoff H. L. Physiological factors affecting transformation of Azotobacter vinelandii. J Bacteriol. 1976 Mar;125(3):1080–1087. doi: 10.1128/jb.125.3.1080-1087.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page W. J., von Tigerstrom M. Induction of transformation competence in Azotobacter vinelandii iron-limited cultures. Can J Microbiol. 1978 Dec;24(12):1590–1594. doi: 10.1139/m78-254. [DOI] [PubMed] [Google Scholar]
- Page W. J., von Tigerstrom M. Optimal conditions for transformation of Azotobacter vinelandii. J Bacteriol. 1979 Sep;139(3):1058–1061. doi: 10.1128/jb.139.3.1058-1061.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravin A. W., Ma M. Specific effects of heating of transformable streptococci on their ability to discriminate between homospecific, heterospecific, and hybrid deoxyribonucleic acid. J Bacteriol. 1972 Feb;109(2):616–625. doi: 10.1128/jb.109.2.616-625.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STUY J. H., STERN D. THE KINETICS OF DNA UPTAKE BY HAEMOPHILUS INFLUENZAE. J Gen Microbiol. 1964 Jun;35:391–400. doi: 10.1099/00221287-35-3-391. [DOI] [PubMed] [Google Scholar]
- Schenk S. P., Earhart C. F. Characterization of the predominant Azotobacter vinelandii envelope protein. J Bacteriol. 1981 Apr;146(1):398–403. doi: 10.1128/jb.146.1.398-403.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp P. A., Sugden B., Sambrook J. Detection of two restriction endonuclease activities in Haemophilus parainfluenzae using analytical agarose--ethidium bromide electrophoresis. Biochemistry. 1973 Jul 31;12(16):3055–3063. doi: 10.1021/bi00740a018. [DOI] [PubMed] [Google Scholar]
- Sisco K. L., Smith H. O. Sequence-specific DNA uptake in Haemophilus transformation. Proc Natl Acad Sci U S A. 1979 Feb;76(2):972–976. doi: 10.1073/pnas.76.2.972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith H. O. Recovery of DNA from gels. Methods Enzymol. 1980;65(1):371–380. doi: 10.1016/s0076-6879(80)65048-4. [DOI] [PubMed] [Google Scholar]
- Stuy J. H. Fate of transforming DNA in the Haemophilus influenzae transformation system. J Mol Biol. 1965 Sep;13(2):554–570. doi: 10.1016/s0022-2836(65)80117-6. [DOI] [PubMed] [Google Scholar]
- Stuy J. H., Van der Have B. Degradation of adsorbed transforming DNA by haemophilus influenzae. J Gen Microbiol. 1971 Feb;65(2):147–152. doi: 10.1099/00221287-65-2-147. [DOI] [PubMed] [Google Scholar]
- Sutrina S. L., Scocca J. J. Haemophilus influenzae periplasmic protein which binds deoxyribonucleic acid: properties and possible participation in genetic transformation. J Bacteriol. 1979 Sep;139(3):1021–1027. doi: 10.1128/jb.139.3.1021-1027.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]