Abstract
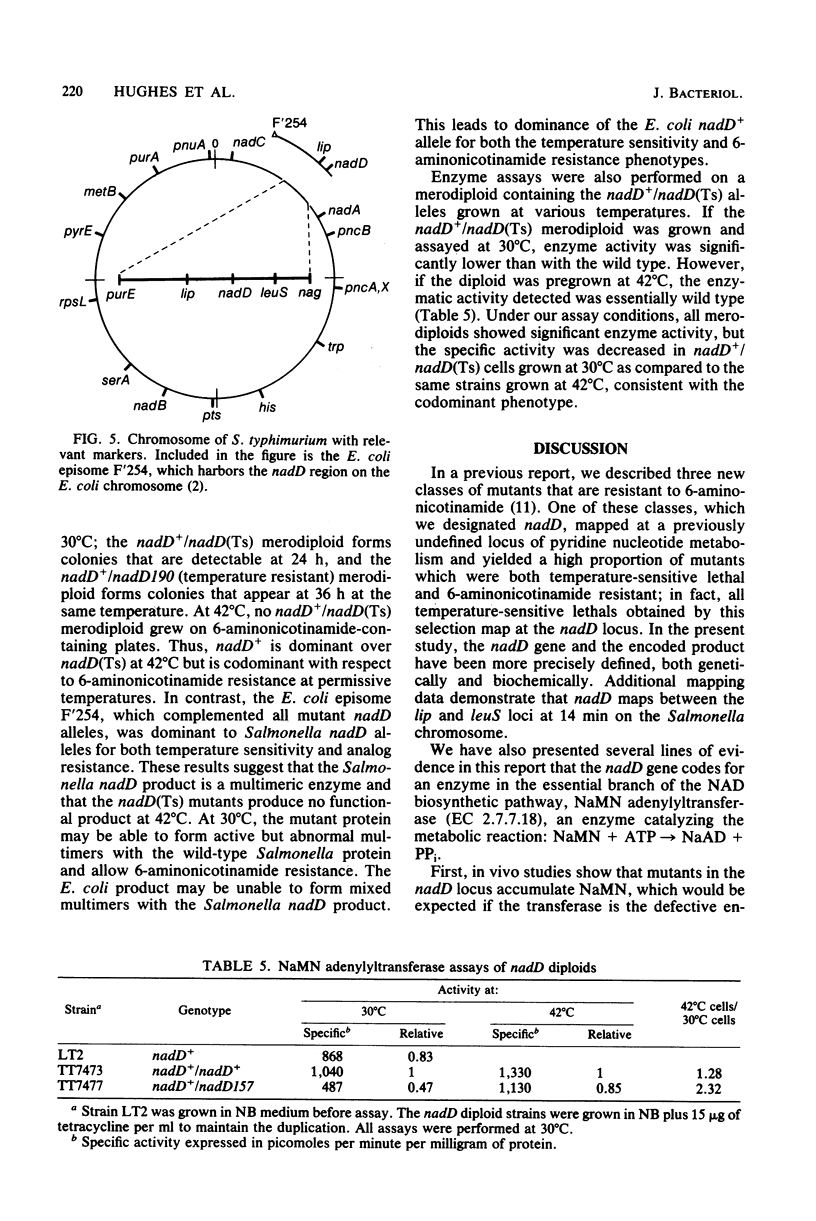
We have located the nadD locus between lip and leuS at 14 min on the Salmonella typhimurium chromosome, and we have shown it to be the structural gene for nicotinic acid mononucleotide adenylyltransferase. This is the first indispensable gene of pyridine nucleotide metabolism that has been identified. Mutants altered at this locus, isolated by their 6-aminonicotinamide resistance phenotype, accumulate abnormally large pools of nicotinic acid mononucleotide in vivo; many exhibit a temperature-sensitive lethal phenotype. Enzyme assays reveal markedly lower transferase activity in mutant extracts than in nadD+ extracts. The partial dominance of nadD mutants when placed in a nadD+/nadD diploid suggests that nicotinic acid mononucleotide adenylyltransferase is a multimeric enzyme.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abstracts of papers presented at the 1980 meetings of the Genetic Society of America. Boulder, Colorado August 18-20, 1980. Genetics. 1980;94(4 Pt 2 Suppl):1–16. [PMC free article] [PubMed] [Google Scholar]
- Anderson R. P., Roth J. R. Tandem chromosomal duplications in Salmonella typhimurium: fusion of histidine genes to novel promoters. J Mol Biol. 1978 Feb 15;119(1):147–166. doi: 10.1016/0022-2836(78)90274-7. [DOI] [PubMed] [Google Scholar]
- Bachmann B. J., Low K. B. Linkage map of Escherichia coli K-12, edition 6. Microbiol Rev. 1980 Mar;44(1):1–56. doi: 10.1128/mr.44.1.1-56.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Calvo J. M., Freundlich M., Umbarger H. E. Regulation of branched-chain amino acid biosynthesis in Salmonella typhimurium: isolation of regulatory mutants. J Bacteriol. 1969 Mar;97(3):1272–1282. doi: 10.1128/jb.97.3.1272-1282.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan R. K., Botstein D., Watanabe T., Ogata Y. Specialized transduction of tetracycline resistance by phage P22 in Salmonella typhimurium. II. Properties of a high-frequency-transducing lysate. Virology. 1972 Dec;50(3):883–898. doi: 10.1016/0042-6822(72)90442-4. [DOI] [PubMed] [Google Scholar]
- Chandler J. L., Gholson R. K. De novo biosynthesis of nicotinamide adenine dinucleotide in Escherichia coli: excretion of quinolinic acid by mutants lacking quinolinate phosphoribosyl transferase. J Bacteriol. 1972 Jul;111(1):98–102. doi: 10.1128/jb.111.1.98-102.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chumley F. G., Menzel R., Roth J. R. Hfr formation directed by tn10. Genetics. 1979 Apr;91(4):639–655. doi: 10.1093/genetics/91.4.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahmen W., Webb B., Preiss J. The deamido-diphosphopyridine nucleotide and diphosphopyridine nucleotide pyrophosphorylases of Escherichia coli and yeast. Arch Biochem Biophys. 1967 May;120(2):440–450. doi: 10.1016/0003-9861(67)90262-7. [DOI] [PubMed] [Google Scholar]
- Foster J. W., Moat A. G. Nicotinamide adenine dinucleotide biosynthesis and pyridine nucleotide cycle metabolism in microbial systems. Microbiol Rev. 1980 Mar;44(1):83–105. doi: 10.1128/mr.44.1.83-105.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes K. T., Cookson B. T., Ladika D., Olivera B. M., Roth J. R. 6-Aminonicotinamide-resistant mutants of Salmonella typhimurium. J Bacteriol. 1983 Jun;154(3):1126–1136. doi: 10.1128/jb.154.3.1126-1136.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth J. R. Frameshift mutations. Annu Rev Genet. 1974;8:319–346. doi: 10.1146/annurev.ge.08.120174.001535. [DOI] [PubMed] [Google Scholar]
- Sanderson K. E., Hartman P. E. Linkage map of Salmonella typhimurium, edition V. Microbiol Rev. 1978 Jun;42(2):471–519. doi: 10.1128/mr.42.2.471-519.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmieger H. A method for detection of phage mutants with altered transducing ability. Mol Gen Genet. 1971;110(4):378–381. doi: 10.1007/BF00438281. [DOI] [PubMed] [Google Scholar]
- Spencer R. L., Preiss J. Biosynthesis of diphosphopyridine nucleotide. The purification and the properties of diphospyridine nucleotide synthetase from Escherichia coli b. J Biol Chem. 1967 Feb 10;242(3):385–392. [PubMed] [Google Scholar]
- VOGEL H. J., BONNER D. M. Acetylornithinase of Escherichia coli: partial purification and some properties. J Biol Chem. 1956 Jan;218(1):97–106. [PubMed] [Google Scholar]


