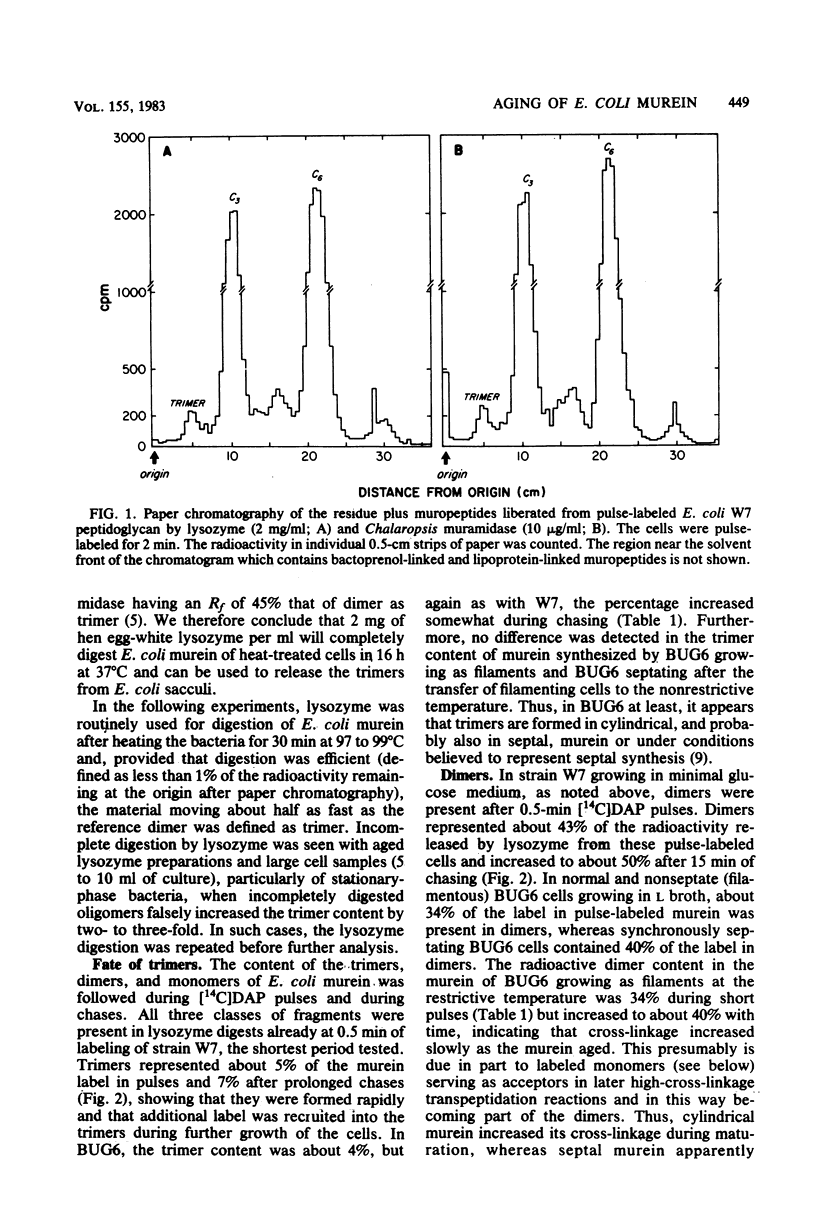
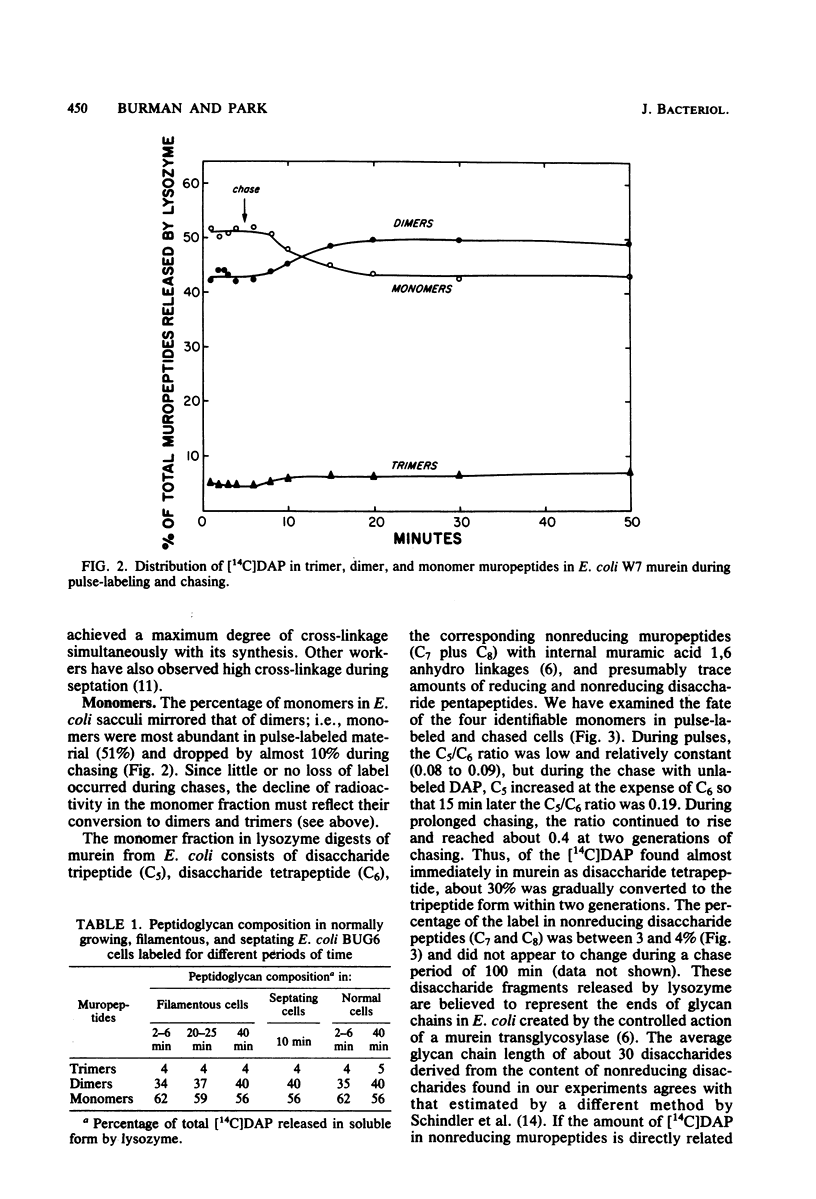
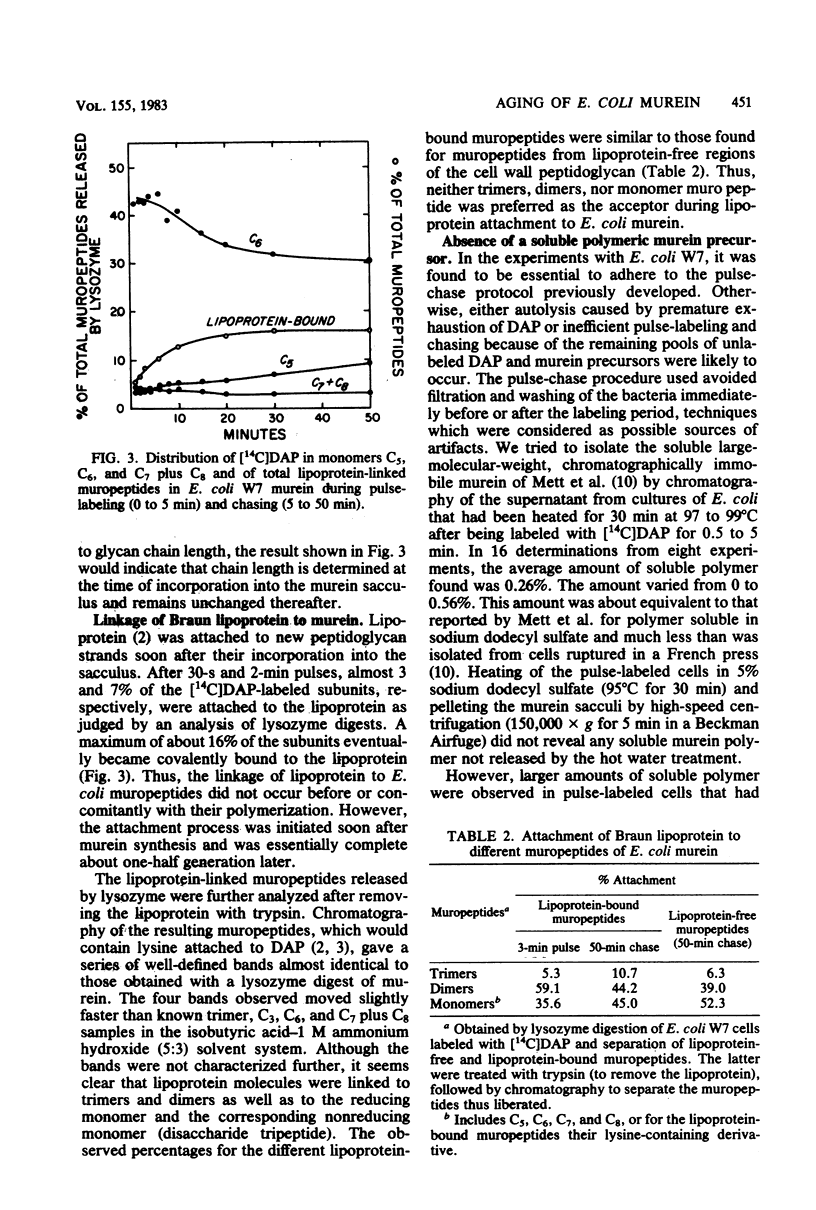
Abstract
Escherichia coli murein was specifically labeled with [14C]diaminopimelic acid in the mutant strains W7 (dap lysA) and BUG6. Pulse-labeled heat-denatured E. coli cells were digested with 2 mg of egg-white lysozyme per ml to degrade the murein completely and free any lipoprotein-bound muropeptide trimers, dimers, and monomers. Pulse-chase experiments showed that the relative percentage of trimers and dimers found in the newly synthesized murein increased somewhat with time at the expense of monomers. The increase in cross-links indicated that the radioactive monomers served as acceptors in multisite transpeptidations occurring after the labeling period. The content of nonreducing monomers (C7 and C8) remained unaltered, indicating that the oligosaccharide chain length did not change with time. A gradual conversion of the reducing disaccharide tetrapeptide monomer to its tripeptide analog occurred during chasing. Braun lipoprotein was linked to about 2% of the murein subunits within 30 s of the incorporation of subunits into insoluble murein, and after one-half a generation of chase, lipoprotein-associated muropeptides had approached the maximum (16% of the total murein subunits). The distribution of muropeptides was similar in lipoprotein-linked and lipoprotein-free murein, showing that the enzyme that links Braun lipoprotein to murein does not discriminate between monomers, dimers, and trimers. No evidence for a chasable, soluble polymer of murein was found in our experiments. Hence, our data support the idea that new murein is incorporated directly into the sacculus without first existing as a soluble intermediate.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beck B. D., Park J. T. Activity of three murein hydrolases during the cell division cycle of Escherichia coli K-12 as measured in toluene-treated cells. J Bacteriol. 1976 Jun;126(3):1250–1260. doi: 10.1128/jb.126.3.1250-1260.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun V., Rehn K. Chemical characterization, spatial distribution and function of a lipoprotein (murein-lipoprotein) of the E. coli cell wall. The specific effect of trypsin on the membrane structure. Eur J Biochem. 1969 Oct;10(3):426–438. doi: 10.1111/j.1432-1033.1969.tb00707.x. [DOI] [PubMed] [Google Scholar]
- Braun V., Wolff H. Attachment of lipoprotein to murein (peptidoglycan) of Escherichia coli in the presence and absence of penicillin FL 1060. J Bacteriol. 1975 Sep;123(3):888–897. doi: 10.1128/jb.123.3.888-897.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Höltje J. V., Mirelman D., Sharon N., Schwarz U. Novel type of murein transglycosylase in Escherichia coli. J Bacteriol. 1975 Dec;124(3):1067–1076. doi: 10.1128/jb.124.3.1067-1076.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LENNOX E. S. Transduction of linked genetic characters of the host by bacteriophage P1. Virology. 1955 Jul;1(2):190–206. doi: 10.1016/0042-6822(55)90016-7. [DOI] [PubMed] [Google Scholar]
- Markiewicz Z., Broome-Smith J. K., Schwarz U., Spratt B. G. Spherical E. coli due to elevated levels of D-alanine carboxypeptidase. Nature. 1982 Jun 24;297(5868):702–704. doi: 10.1038/297702a0. [DOI] [PubMed] [Google Scholar]
- Mett H., Bracha R., Mirelman D. Soluble nascent peptidoglycan in growing Escherichia coli cells. J Biol Chem. 1980 Oct 25;255(20):9884–9890. [PubMed] [Google Scholar]
- Reeve J. N., Groves D. J., Clark D. J. Regulation of Cell Division in Escherichia coli: Characterization of Temperature-Sensitive Division Mutants. J Bacteriol. 1970 Dec;104(3):1052–1064. doi: 10.1128/jb.104.3.1052-1064.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryter A., Hirota Y., Schwarz U. Process of cellular division in Escherichia coli growth pattern of E. coli murein. J Mol Biol. 1973 Jun 25;78(1):185–195. doi: 10.1016/0022-2836(73)90437-3. [DOI] [PubMed] [Google Scholar]
- Schindler M., Mirelman D., Schwarz U. Quantitative determination of N-acetylglucosamine residues at the non-reducing ends of peptidoglycan chains by enzymic attachment of [14C]-D-galactose. Eur J Biochem. 1976 Dec;71(1):131–134. doi: 10.1111/j.1432-1033.1976.tb11098.x. [DOI] [PubMed] [Google Scholar]
- Verwer R. W., Nanninga N. Pattern of meso-dl-2,6-diaminopimelic acid incorporation during the division cycle of Escherichia coli. J Bacteriol. 1980 Oct;144(1):327–336. doi: 10.1128/jb.144.1.327-336.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WEIDEL W., PELZER H. BAGSHAPED MACROMOLECULES--A NEW OUTLOOK ON BACTERIAL CELL WALLS. Adv Enzymol Relat Areas Mol Biol. 1964;26:193–232. doi: 10.1002/9780470122716.ch5. [DOI] [PubMed] [Google Scholar]
- de Pedro M. A., Schwarz U. Heterogeneity of newly inserted and preexisting murein in the sacculus of Escherichia coli. Proc Natl Acad Sci U S A. 1981 Sep;78(9):5856–5860. doi: 10.1073/pnas.78.9.5856. [DOI] [PMC free article] [PubMed] [Google Scholar]


