Abstract
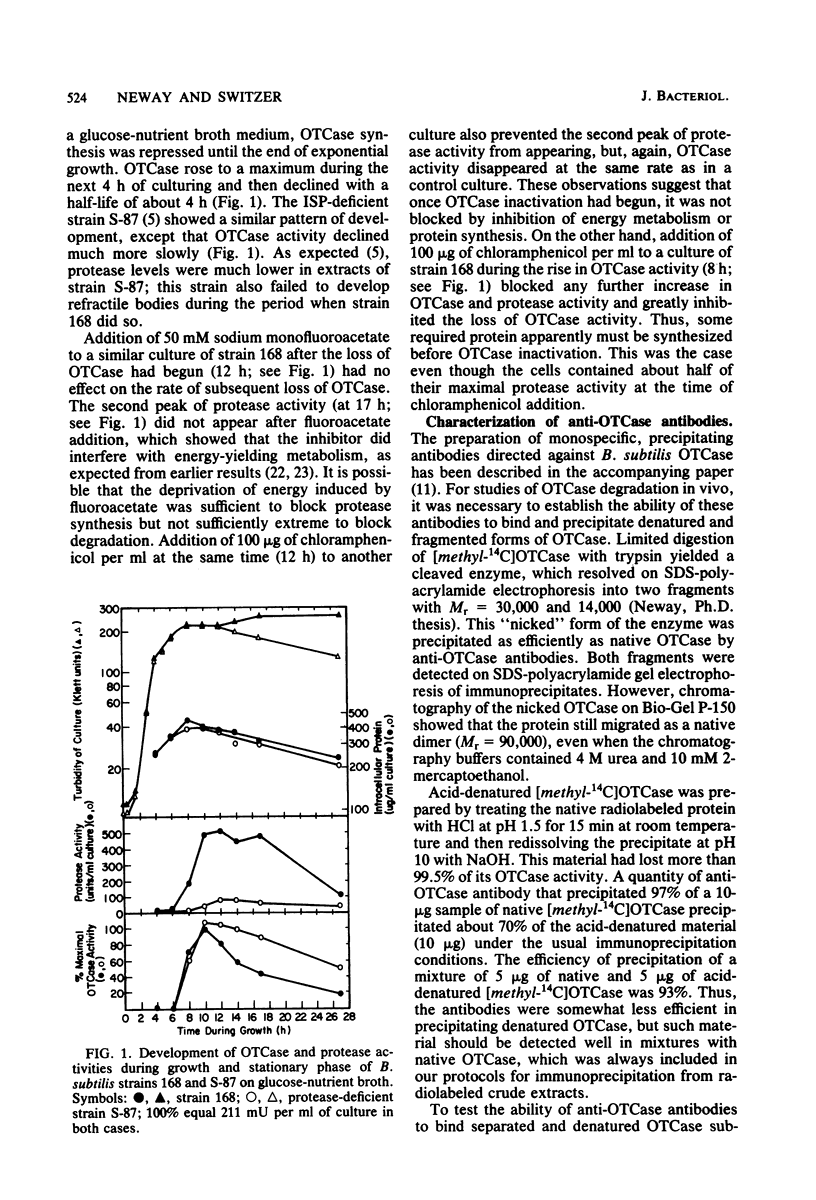
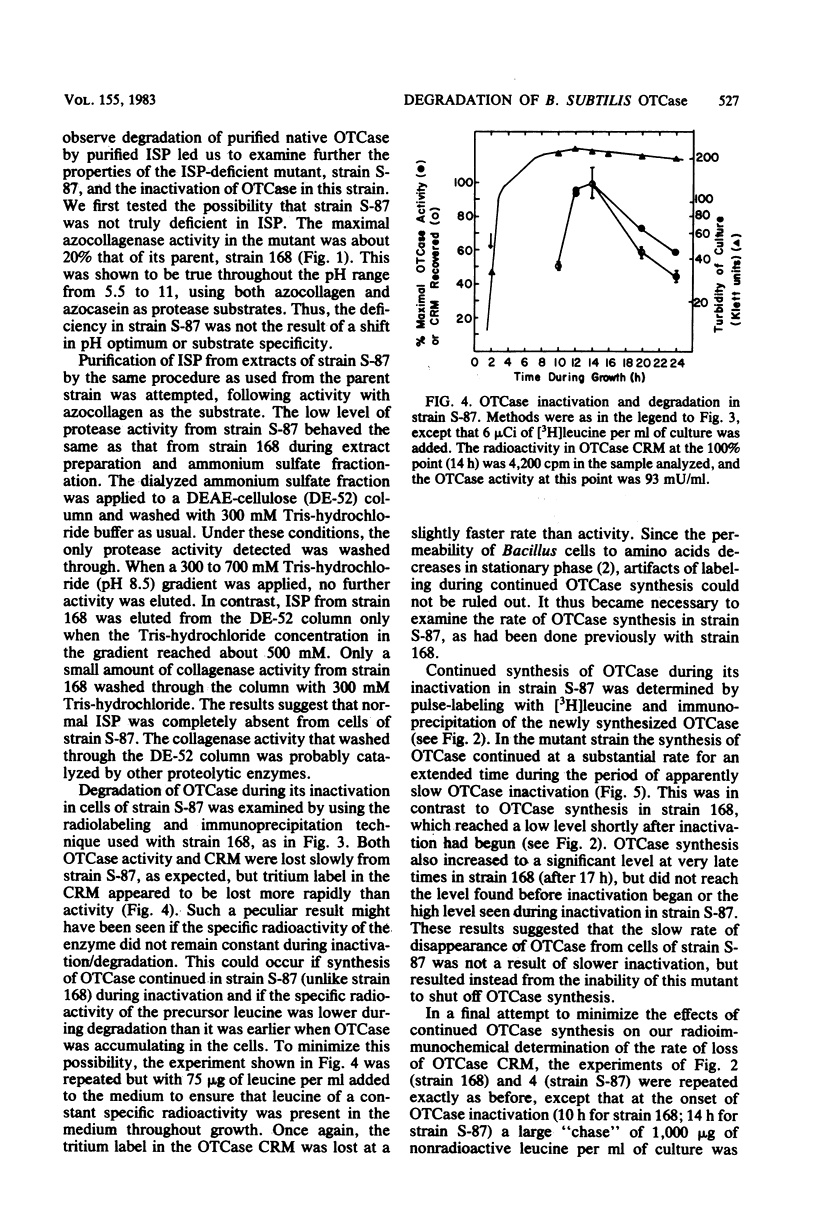
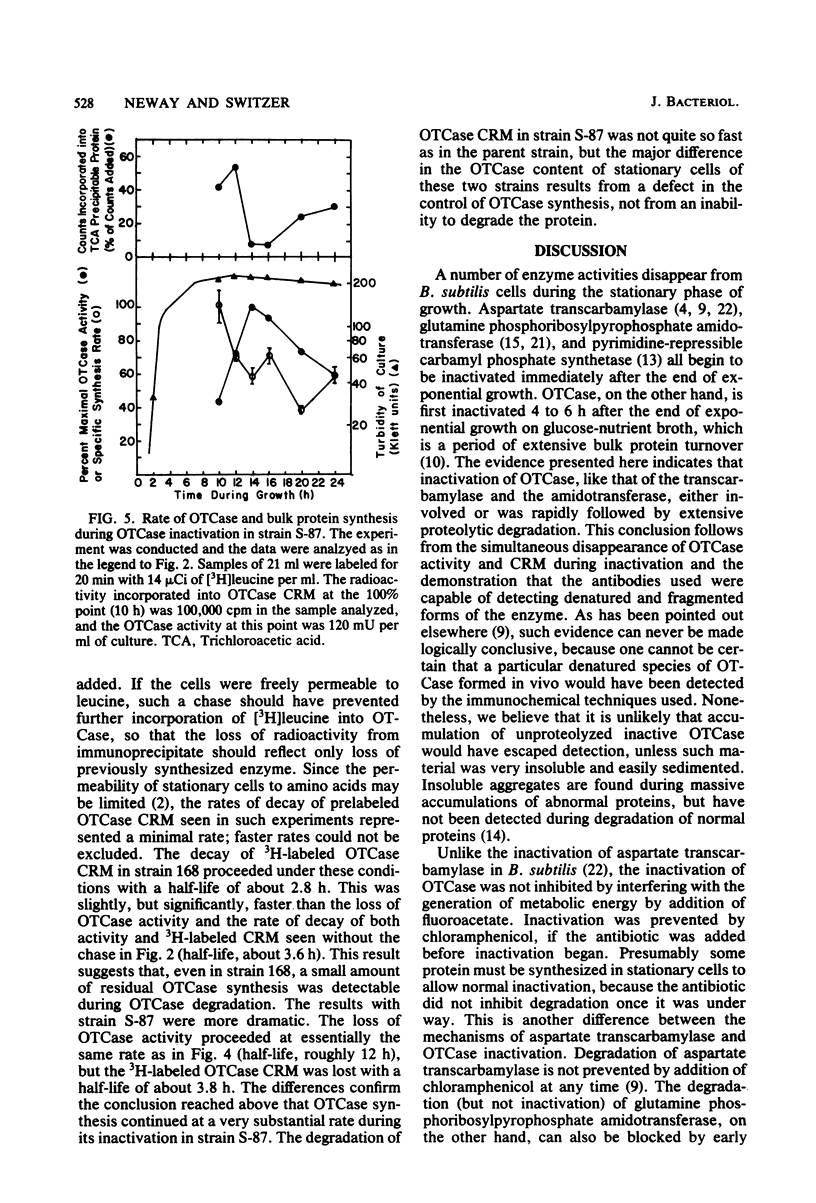
When Bacillus subtilis cells grew and sporulated on glucose-nutrient broth, ornithine transcarbamylase (OTCase) was synthesized in the early stationary phase and then inactivated. The loss of OTCase activity was much slower in a mutant that was deficient in a major intracellular serine protease (ISP). Immunochemical analysis showed that synthesis of OTCase decreased to a low, but detectable, level during its inactivation and that loss of activity was paralleled by loss of cross-reactive protein. Because the antibodies were capable of detecting denatured and fragmented forms of OTCase, we conclude that inactivation involved or was rapidly followed by degradation in vivo. Native OTCase was not degraded in crude extracts or when purified ISP and OTCase were incubated together under a variety of conditions. Synthesis of OTCase was not shut off normally in the ISP-deficient mutant. When the effects of continued synthesis were minimized, OTCase was degraded only slightly slower in the mutant than in its parent. Thus, the mutant had unanticipated pleiotropic characteristics, and it was unlikely that ISP played a major role in the degradation of OTCase in vivo.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson L. E., McClure W. O. An improved scintillation cocktail of high-solubilizing power. Anal Biochem. 1973 Jan;51(1):173–179. doi: 10.1016/0003-2697(73)90465-x. [DOI] [PubMed] [Google Scholar]
- Burnette W. N. "Western blotting": electrophoretic transfer of proteins from sodium dodecyl sulfate--polyacrylamide gels to unmodified nitrocellulose and radiographic detection with antibody and radioiodinated protein A. Anal Biochem. 1981 Apr;112(2):195–203. doi: 10.1016/0003-2697(81)90281-5. [DOI] [PubMed] [Google Scholar]
- Deutscher M. P., Kornberg A. Biochemical studies of bacterial sporulation and germination. 8. Patterns of enzyme development during growth and sporulation of Baccillus subtilis. J Biol Chem. 1968 Sep 25;243(18):4653–4660. [PubMed] [Google Scholar]
- Hageman J. H., Carlton B. C. Effects of mutational loss of specific intracellular proteases on the sporulation of Bacillus subtilis. J Bacteriol. 1973 May;114(2):612–617. doi: 10.1128/jb.114.2.612-617.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jentoft N., Dearborn D. G. Labeling of proteins by reductive methylation using sodium cyanoborohydride. J Biol Chem. 1979 Jun 10;254(11):4359–4365. [PubMed] [Google Scholar]
- Kerjan P., Keryer E., Szulmajster J. Characterization of a thermosensitive sporulation mutant of Bacillus subtilis affected in the structural gene of an intracellular protease. Eur J Biochem. 1979 Aug 1;98(2):353–362. doi: 10.1111/j.1432-1033.1979.tb13194.x. [DOI] [PubMed] [Google Scholar]
- Maurizi M. R., Brabson J. S., Switzer R. L. Immunochemical studies of the inactivation of aspartate transcarbamylase by stationary phase Bacillus subtilis cells. Evidence for selective, energy-dependent degradation. J Biol Chem. 1978 Aug 25;253(16):5585–5593. [PubMed] [Google Scholar]
- Maurizi M. R., Switzer R. L. Proteolysis in bacterial sporulation. Curr Top Cell Regul. 1980;16:163–224. doi: 10.1016/b978-0-12-152816-4.50010-8. [DOI] [PubMed] [Google Scholar]
- Neway J. O., Switzer R. L. Purification, characterization, and physiological function of Bacillus subtilis ornithine transcarbamylase. J Bacteriol. 1983 Aug;155(2):512–521. doi: 10.1128/jb.155.2.512-521.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulus T. J., Switzer R. L. Synthesis and inactivation of carbamyl phosphate synthetase isozymes of Bacillus subtilis during growth and sporulation. J Bacteriol. 1979 Dec;140(3):769–773. doi: 10.1128/jb.140.3.769-773.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruppen M. E., Switzer R. L. Degradation of Bacillus subtilis glutamine phosphoribosylpyrophosphate amidotransferase in vivo. J Biol Chem. 1983 Mar 10;258(5):2843–2851. [PubMed] [Google Scholar]
- Setlow P., Primus G. Protein metabolism during germination of Bacillus megaterium spores. I. Protein synthesis and amino acid metabolism. J Biol Chem. 1975 Jan 25;250(2):623–630. [PubMed] [Google Scholar]
- Stepanov V. M., Strongin A. Y., Izotova L. S., Abramov Z. T., Lyublinskaya L. A., Ermakova L. M., Baratova L. A., Belyanova L. P. Intracellular serine protease from Bacillus subtilis. Structural comparison with extracellular serine proteases-subtilisins. Biochem Biophys Res Commun. 1977 Jul 11;77(1):298–305. doi: 10.1016/s0006-291x(77)80196-4. [DOI] [PubMed] [Google Scholar]
- Strongin A. Y., Gorodetsky D. I., Kuznetsova I. A., Yanonis V. V., Abramov Z. T., Belyanova L. P., Baratova L. A., Stepanov V. M. Intracellular serine proteinase of Bacillus subtilis strain Marburg 168. Comparison with the homologous enzyme from Bacillus subtilis strain A-50. Biochem J. 1979 May 1;179(2):333–339. doi: 10.1042/bj1790333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strongin A. Y., Izotova L. S., Abramov Z. T., Gorodetsky D. I., Ermakova L. M., Baratova L. A., Belyanova L. P., Stepanov V. M. Intracellular serine protease of Bacillus subtilis: sequence homology with extracellular subtilisins. J Bacteriol. 1978 Mar;133(3):1401–1411. doi: 10.1128/jb.133.3.1401-1411.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Switzer R. L. The inactivation of microbial enzymes in vivo. Annu Rev Microbiol. 1977;31:135–157. doi: 10.1146/annurev.mi.31.100177.001031. [DOI] [PubMed] [Google Scholar]
- Turnbough C. L., Jr, Switzer R. L. Oxygen-dependent inactivation of glutamine phosphoribosylpyrophosphate amidotransferase in stationary-phase cultures of Bacillus subtilis. J Bacteriol. 1975 Jan;121(1):108–114. doi: 10.1128/jb.121.1.108-114.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waindle L. M., Switzer R. L. Inactivation of aspartic transcarbamylase in sporulating Bacillus subtilis: demonstration of a requirement for metabolic energy. J Bacteriol. 1973 May;114(2):517–527. doi: 10.1128/jb.114.2.517-527.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yousten A. A., Hanson R. S. Sporulation of tricarboxylic acid cycle mutants of Bacillus subtilis. J Bacteriol. 1972 Feb;109(2):886–894. doi: 10.1128/jb.109.2.886-894.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]


