Abstract
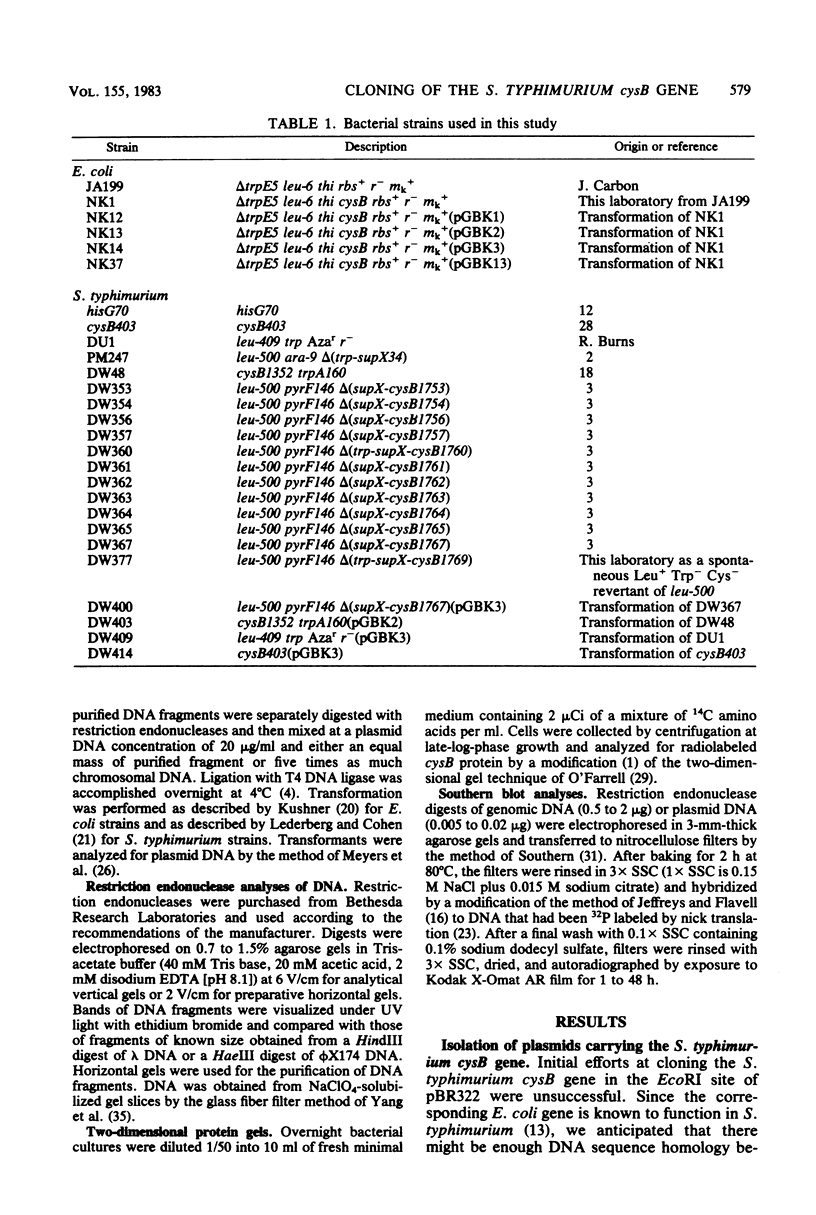
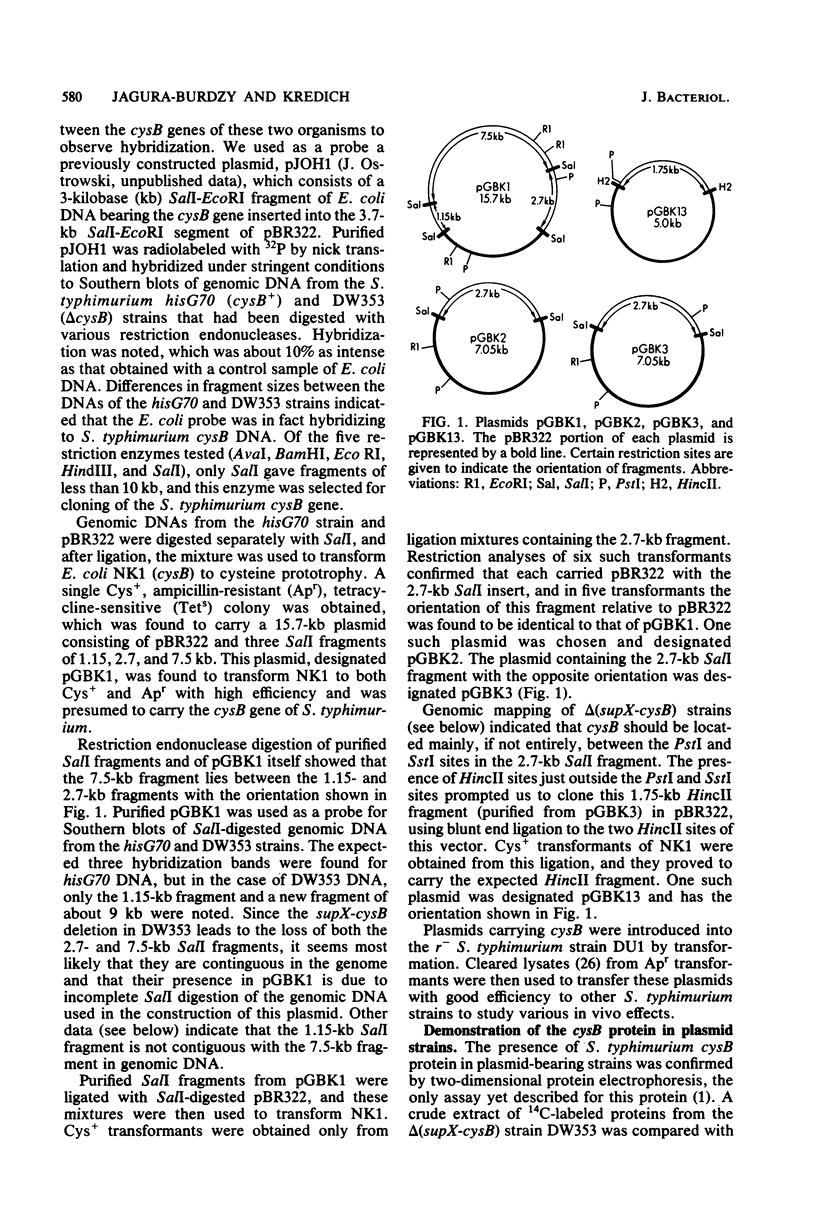
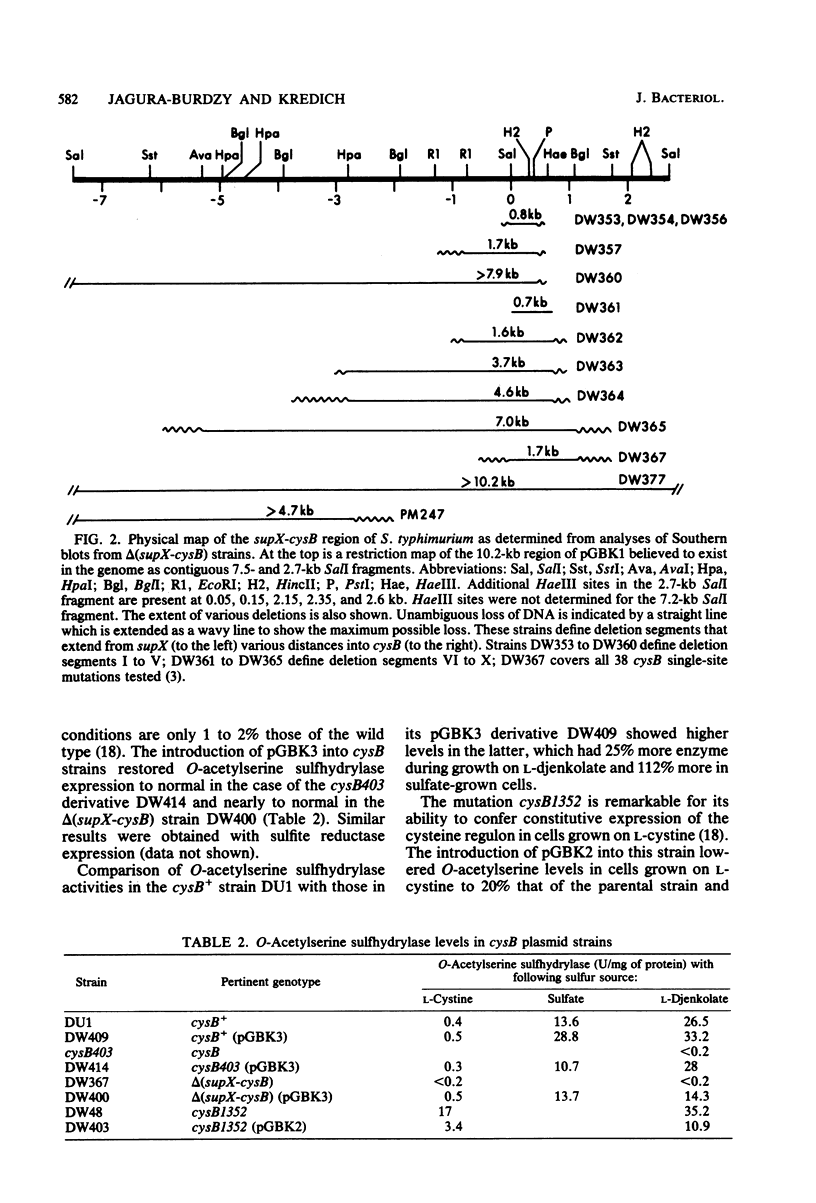
The cysB region of Salmonella typhimurium was cloned in pBR322 and localized to a 1.75-kilobase HincII fragment. Two-dimensional protein electropherograms showed levels of the cysB polypeptide chain that were several fold higher in plasmid-bearing strains than in the wild type. Fully derepressed levels of sulfite reductase and O-acetylserine sulfhydrylase in cysB plasmid-bearing strains were only 25% higher than in the wild type, suggesting that the product of this regulatory gene ordinarily is not a limiting factor in the expression of the cysteine regulon. The mapping of cysB deletions by Southern blots showed a good correlation between the genetic and the physical maps of this gene. The supX gene was initially cloned with cysB and is within 0.7 kilobase of cysB.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baptist E. W., Hallquist S. G., Kredich N. M. Identification of the Salmonella typhimurium cysB gene product by two-dimensional protein electrophoresis. J Bacteriol. 1982 Jul;151(1):495–499. doi: 10.1128/jb.151.1.495-499.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauerle R. H., Margolin P. Evidence for two sites for initiation of gene expression in the tryptophan operon of Salmonella typhimurium. J Mol Biol. 1967 Jun 28;26(3):423–436. doi: 10.1016/0022-2836(67)90313-0. [DOI] [PubMed] [Google Scholar]
- Cheney R. W., Jr, Kredich N. M. Fine-structure genetic map of the cysB locus in Salmonella typhimurium. J Bacteriol. 1975 Dec;124(3):1273–1281. doi: 10.1128/jb.124.3.1273-1281.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins C. J., Jackson D. A., DeVries A. J. Biochemical construction of specific chimeric plasmids from ColE1 DNA and unfractionated Escherichia coli DNA. Proc Natl Acad Sci U S A. 1976 Nov;73(11):3838–3842. doi: 10.1073/pnas.73.11.3838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DEVITO P. C., DREYFUSS J. METABOLIC REGULATION OF ADENOSINE TRIPHOSPHATE SULFURYLASE IN YEAST. J Bacteriol. 1964 Nov;88:1341–1348. doi: 10.1128/jb.88.5.1341-1348.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiNardo S., Voelkel K. A., Sternglanz R., Reynolds A. E., Wright A. Escherichia coli DNA topoisomerase I mutants have compensatory mutations in DNA gyrase genes. Cell. 1982 Nov;31(1):43–51. doi: 10.1016/0092-8674(82)90403-2. [DOI] [PubMed] [Google Scholar]
- Dubnau E., Lenny A. B., Margolin P. Nonsense mutations of the supX locus: further characterization of the supX mutant phenotype. Mol Gen Genet. 1973 Nov 12;126(3):191–200. doi: 10.1007/BF00267530. [DOI] [PubMed] [Google Scholar]
- Dubnau E., Margolin P. Suppression of promoter mutations by the pleiotropic supx mutations. Mol Gen Genet. 1972;117(2):91–112. doi: 10.1007/BF00267607. [DOI] [PubMed] [Google Scholar]
- Graf L. H., Jr, Burns R. O. The supX-leu-500 mutations and expression of the leucine operon. Mol Gen Genet. 1973 Nov 22;126(4):291–301. doi: 10.1007/BF00269439. [DOI] [PubMed] [Google Scholar]
- Hartman P. E., Hartman Z., Stahl R. C. Classification and mapping of spontaneous and induced mutations in the histidine operon of Salmonella. Adv Genet. 1971;16:1–34. doi: 10.1016/s0065-2660(08)60352-1. [DOI] [PubMed] [Google Scholar]
- Ino I., Demerec M. Enteric hybrids. II. S. typhimurium-E. coli hybrids for the trp-cysB-pyrF region. Genetics. 1968 Jun;59(2):167–176. doi: 10.1093/genetics/59.2.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jagura G., Hulanicka D. Analysis of merodiploids of the cysB region in Salmonella typhimurium. Mol Gen Genet. 1978 Sep 20;165(1):31–38. doi: 10.1007/BF00270373. [DOI] [PubMed] [Google Scholar]
- Jones-Mortimer M. C. Positive control of sulphate reduction in Escherichia coli. The nature of the pleiotropic cysteineless mutants of E. coli K12. Biochem J. 1968 Dec;110(3):597–602. doi: 10.1042/bj1100597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kredich N. M. Regulation of L-cysteine biosynthesis in Salmonella typhimurium. I. Effects of growth of varying sulfur sources and O-acetyl-L-serine on gene expression. J Biol Chem. 1971 Jun 10;246(11):3474–3484. [PubMed] [Google Scholar]
- Kupersztoch-Portnoy Y. M., Lovett M. A., Helinski D. R. Strand and site specificity of the relaxation event for the relaxation complex of the antibiotic resistance plasmid R6K. Biochemistry. 1974 Dec 31;13(27):5484–5490. doi: 10.1021/bi00724a005. [DOI] [PubMed] [Google Scholar]
- Lederberg E. M., Cohen S. N. Transformation of Salmonella typhimurium by plasmid deoxyribonucleic acid. J Bacteriol. 1974 Sep;119(3):1072–1074. doi: 10.1128/jb.119.3.1072-1074.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenny A. B., Margolin P. Locations of the opp and supX genes of Salmonella typhimurium and Escherichia coli. J Bacteriol. 1980 Aug;143(2):747–752. doi: 10.1128/jb.143.2.747-752.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maniatis T., Kee S. G., Efstratiadis A., Kafatos F. C. Amplification and characterization of a beta-globin gene synthesized in vitro. Cell. 1976 Jun;8(2):163–182. doi: 10.1016/0092-8674(76)90001-5. [DOI] [PubMed] [Google Scholar]
- Mascarenhas D. M., Yudkin M. D. Identification of a positive regulatory protein in Escherichia coli: the product of the cysB gene. Mol Gen Genet. 1980 Feb;177(3):535–539. doi: 10.1007/BF00271494. [DOI] [PubMed] [Google Scholar]
- Meyers J. A., Sanchez D., Elwell L. P., Falkow S. Simple agarose gel electrophoretic method for the identification and characterization of plasmid deoxyribonucleic acid. J Bacteriol. 1976 Sep;127(3):1529–1537. doi: 10.1128/jb.127.3.1529-1537.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizobuchi K, Demerec M, Gillespie D H. Cysteine Mutants of Salmonella Typhimurium. Genetics. 1962 Nov;47(11):1617–1627. doi: 10.1093/genetics/47.11.1617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Pruss G. J., Manes S. H., Drlica K. Escherichia coli DNA topoisomerase I mutants: increased supercoiling is corrected by mutations near gyrase genes. Cell. 1982 Nov;31(1):35–42. doi: 10.1016/0092-8674(82)90402-0. [DOI] [PubMed] [Google Scholar]
- Southern E. Gel electrophoresis of restriction fragments. Methods Enzymol. 1979;68:152–176. doi: 10.1016/0076-6879(79)68011-4. [DOI] [PubMed] [Google Scholar]
- Stuttard C. Tryptophan biosynthesis in Salmonella typhimurium: location in trpB of a genetic difference between strains LT2 and LT7. J Bacteriol. 1975 Sep;123(3):878–887. doi: 10.1128/jb.123.3.878-887.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tully M., Yudkin M. D. Fine-structure mapping and complementation analysis of the Escherichia coli cysB gene. J Bacteriol. 1977 Jul;131(1):49–56. doi: 10.1128/jb.131.1.49-56.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VOGEL H. J., BONNER D. M. Acetylornithinase of Escherichia coli: partial purification and some properties. J Biol Chem. 1956 Jan;218(1):97–106. [PubMed] [Google Scholar]
- Yang R., Lis J., Wu R. Elution of DNA from agarose gels after electrophoresis. Methods Enzymol. 1979;68:176–182. doi: 10.1016/0076-6879(79)68012-6. [DOI] [PubMed] [Google Scholar]


