Abstract
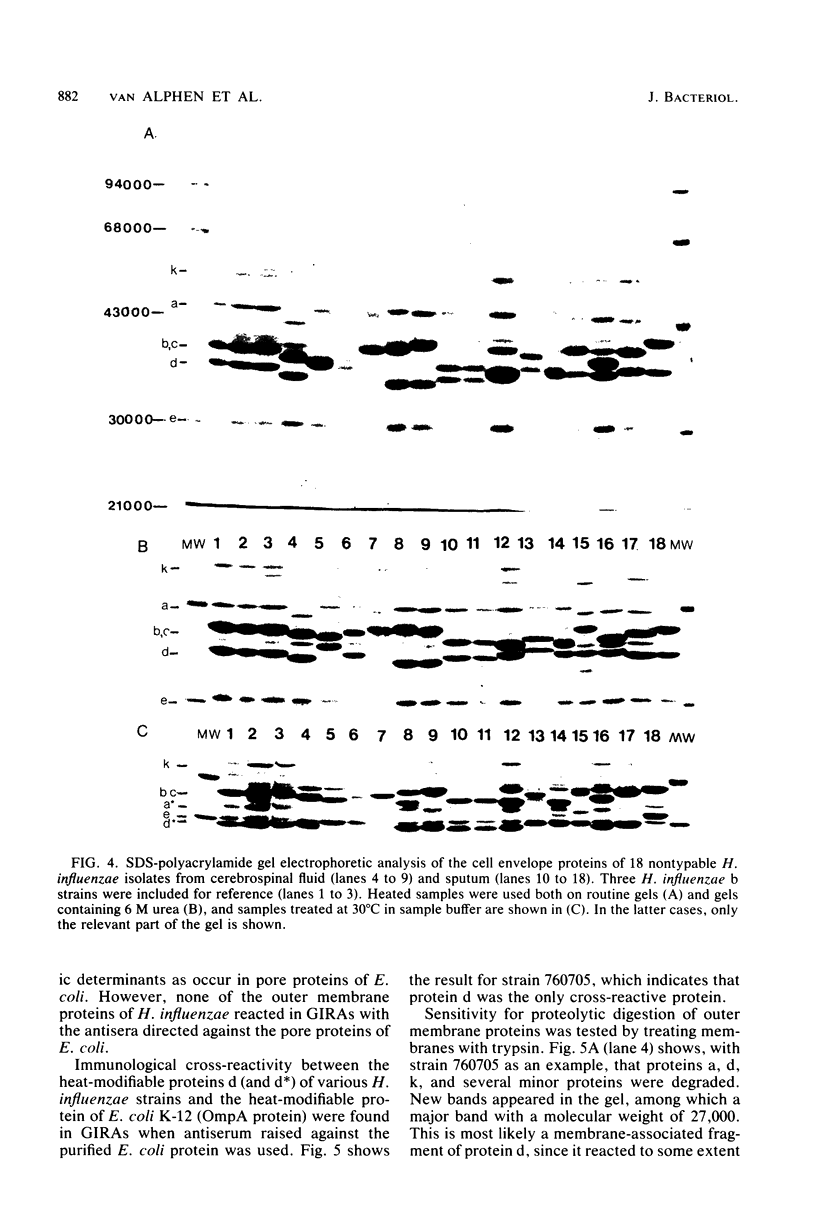
Several properties of Haemophilus influenzae outer membrane proteins were analyzed to define related proteins in various isolates. H. influenzae type b 760705 had six major outer membrane proteins with the following characteristics. Protein a (Mr, 47,000) demonstrated heat modifiability in sodium dodecyl sulfate; its apparent molecular weight was 34,000 at temperatures below 60 degrees C. This protein was extracted from cell envelopes by using Triton X-100-10 mM MgCl2; in cell envelope preparations, the protein was degraded by trypsin. Proteins b (Mr, 41,000) and c (Mr, 40,000) were insensitive to trypsin degradation, were not heat modifiable in sodium dodecyl sulfate, and were peptidoglycan associated in 0.5% Triton X-100-0.2% sodium dodecyl sulfate. The amount of protein b was reduced in ultrasonically obtained cell envelopes. Protein d (Mr, 37,000) was heat modifiable in sodium dodecyl sulfate with an Mr of 28,000 at temperatures below 100 degrees C and was degraded by trypsin, leaving a membrane-bound fragment of Mr, 27,000. Both the intact and degraded proteins were immunologically cross-reactive with the heat-modifiable OmpA protein of Escherichia coli K-12. Protein d was absent in LiCl-EDTA extracts of cells. Protein e (Mr, 30,000), invariably present in all H. influenzae strains tested, was insensitive to trypsin and absent in LiCl-EDTA extracts of cells. Protein k (Mr, 58,000) was extracted from cell envelopes with 2% Triton X-100-10 mM MgCl2 and, in cell envelopes, appeared to be sensitive to trypsin degradation. Proteins with similar properties to those of proteins a to k were found in 10 other H. influenzae b strains, reference strains with serotype a, c, d, e, and f capsules, and 18 of 20 nonencapsulated strains. Their relative molecular weights, however, varied.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson P., Insel R. A., Smith D. H., Cate T. R., Couch R. B., Glezen W. P. A polysaccharide-protein complex from Haemophilus influenzae type b. III. Vaccine trial in human adults. J Infect Dis. 1981 Dec;144(6):530–538. doi: 10.1093/infdis/144.6.530. [DOI] [PubMed] [Google Scholar]
- Barenkamp S. J., Munson R. S., Jr, Granoff D. M. Comparison of outer-membrane protein subtypes and biotypes of isolates of Haemophilus influenzae type b. J Infect Dis. 1981 Nov;144(5):480–480. doi: 10.1093/infdis/144.5.480. [DOI] [PubMed] [Google Scholar]
- Barenkamp S. J., Munson R. S., Jr, Granoff D. M. Subtyping isolates of Haemophilus influenzae type b by outer-membrane protein profiles. J Infect Dis. 1981 May;143(5):668–676. doi: 10.1093/infdis/143.5.668. [DOI] [PubMed] [Google Scholar]
- Beher M. G., Schnaitman C. A., Pugsley A. P. Major heat-modifiable outer membrane protein in gram-negative bacteria: comparison with the ompA protein of Escherichia coli. J Bacteriol. 1980 Aug;143(2):906–913. doi: 10.1128/jb.143.2.906-913.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crisel R. M., Baker R. S., Dorman D. E. Capsular polymer of Haemophilus influenzae, type b. I. Structural characterization of the capsular polymer of strain Eagan. J Biol Chem. 1975 Jul 10;250(13):4926–4930. [PubMed] [Google Scholar]
- Dirks-Go S. I., Zanen H. C. Latex agglutination, counterimmunoelectrophoresis, and protein A co-agglutination in diagnosis of bacterial meningitis. J Clin Pathol. 1978 Dec;31(12):1167–1171. doi: 10.1136/jcp.31.12.1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frasch C. E., Gotschlich E. C. An outer membrane protein of Neisseria meningitidis group B responsible for serotype specificity. J Exp Med. 1974 Jul 1;140(1):87–104. doi: 10.1084/jem.140.1.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hancock R. E., Nikaido H. Outer membranes of gram-negative bacteria. XIX. Isolation from Pseudomonas aeruginosa PAO1 and use in reconstitution and definition of the permeability barrier. J Bacteriol. 1978 Oct;136(1):381–390. doi: 10.1128/jb.136.1.381-390.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilian M. A taxonomic study of the genus Haemophilus, with the proposal of a new species. J Gen Microbiol. 1976 Mar;93(1):9–62. doi: 10.1099/00221287-93-1-9. [DOI] [PubMed] [Google Scholar]
- Loeb M. R., Smith D. H. Outer membrane protein composition in disease isolates of Haemophilus influenzae: pathogenic and epidemiological implications. Infect Immun. 1980 Dec;30(3):709–717. doi: 10.1128/iai.30.3.709-717.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loeb M. R., Zachary A. L., Smith D. H. Isolation and partial characterization of outer and inner membranes from encapsulated Haemophilus influenzae type b. J Bacteriol. 1981 Jan;145(1):596–604. doi: 10.1128/jb.145.1.596-604.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lugtenberg B., Bronstein H., van Selm N., Peters R. Peptidoglycan-associated outer membrane proteins in gammegatine bacteria. Biochim Biophys Acta. 1977 Mar 17;465(3):571–578. doi: 10.1016/0005-2736(77)90274-7. [DOI] [PubMed] [Google Scholar]
- Lugtenberg B., Meijers J., Peters R., van der Hoek P., van Alphen L. Electrophoretic resolution of the "major outer membrane protein" of Escherichia coli K12 into four bands. FEBS Lett. 1975 Oct 15;58(1):254–258. doi: 10.1016/0014-5793(75)80272-9. [DOI] [PubMed] [Google Scholar]
- Moxon E. R., Vaughn K. A. The type b capsular polysaccharide as a virulence determinant of Haemophilus influenzae: studies using clinical isolates and laboratory transformants. J Infect Dis. 1981 Apr;143(4):517–524. doi: 10.1093/infdis/143.4.517. [DOI] [PubMed] [Google Scholar]
- Osborn M. J., Gander J. E., Parisi E., Carson J. Mechanism of assembly of the outer membrane of Salmonella typhimurium. Isolation and characterization of cytoplasmic and outer membrane. J Biol Chem. 1972 Jun 25;247(12):3962–3972. [PubMed] [Google Scholar]
- Overbeeke N., Lugtenberg B. Major outer membrane proteins of Escherichia coli strains of human origin. J Gen Microbiol. 1980 Dec;121(2):373–380. doi: 10.1099/00221287-121-2-373. [DOI] [PubMed] [Google Scholar]
- Peltola H., Käyhty H., Sivonen A., Mäkelä H. Haemophilus influenzae type b capsular polysaccharide vaccine in children: a double-blind field study of 100,000 vaccinees 3 months to 5 years of age in Finland. Pediatrics. 1977 Nov;60(5):730–737. [PubMed] [Google Scholar]
- Roberts M., Stull T. L., Smith A. L. Comparative virulence of Haemophilus influenzae with a type b or type d capsule. Infect Immun. 1981 May;32(2):518–524. doi: 10.1128/iai.32.2.518-524.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson S. M., Frisch C. F., Gulig P. A., Kettman J. R., Johnston K. H., Hansen E. J. Monoclonal antibodies directed against a cell surface-exposed outer membrane protein of Haemophilus influenzae type b. Infect Immun. 1982 Apr;36(1):80–88. doi: 10.1128/iai.36.1.80-88.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnaitman C. A. Effect of ethylenediaminetetraacetic acid, Triton X-100, and lysozyme on the morphology and chemical composition of isolate cell walls of Escherichia coli. J Bacteriol. 1971 Oct;108(1):553–563. doi: 10.1128/jb.108.1.553-563.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutton A., Schneerson R., Kendall-Morris S., Robbins J. B. Differential complement resistance mediates virulence of Haemophilus influenzae type b. Infect Immun. 1982 Jan;35(1):95–104. doi: 10.1128/iai.35.1.95-104.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Heerikhuizen H., Kwak E., van Bruggen E. F., Witholt B. Characterization of a low density cytoplasmic membrane subfraction isolated from Escherichia coli. Biochim Biophys Acta. 1975 Dec 1;413(2):177–191. doi: 10.1016/0005-2736(75)90102-9. [DOI] [PubMed] [Google Scholar]