Abstract
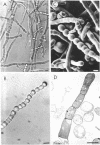
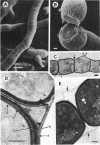
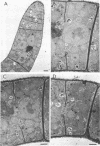
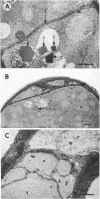
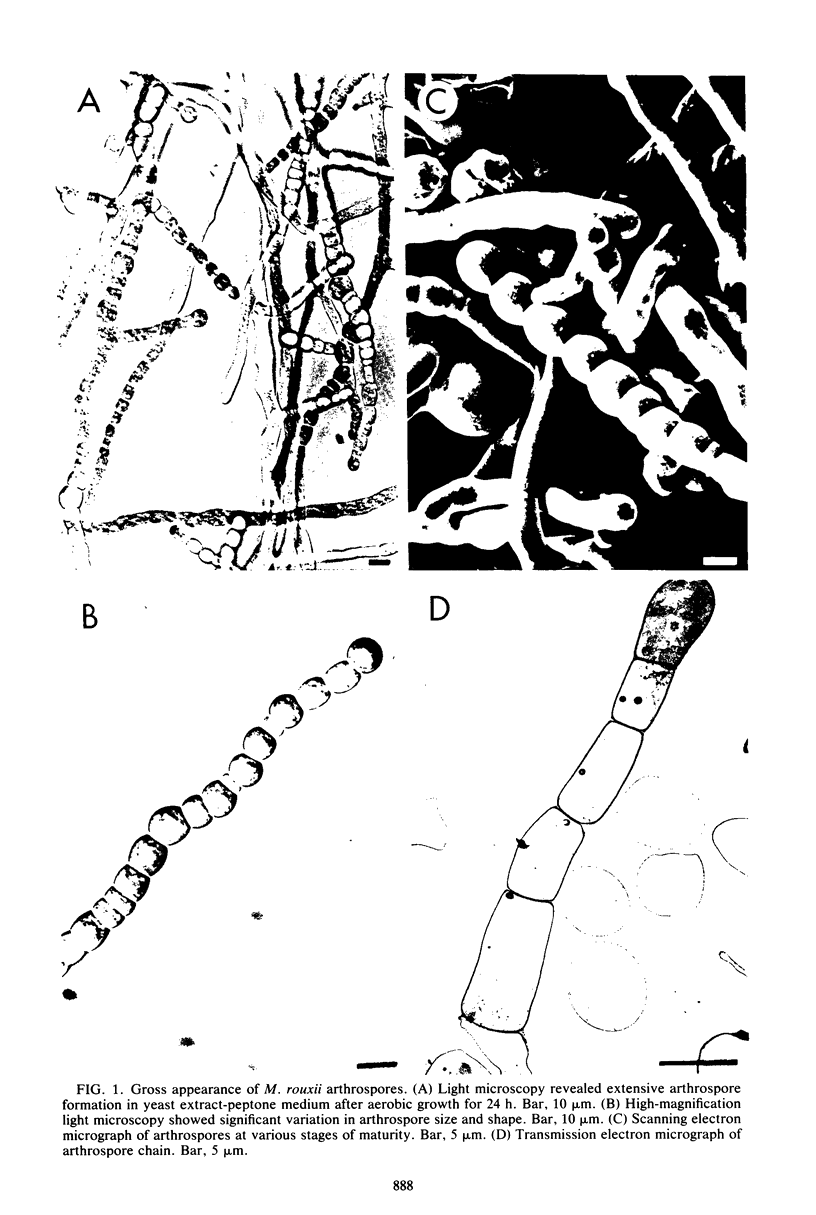
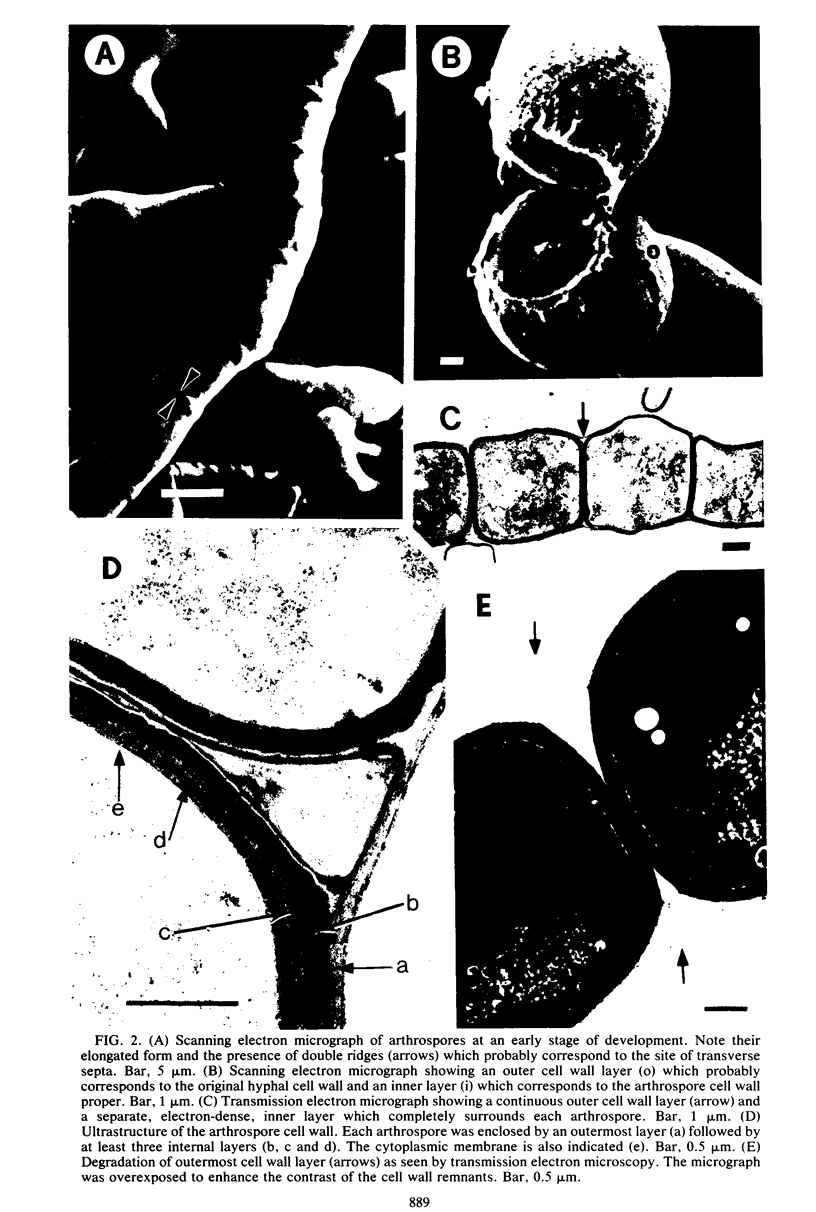
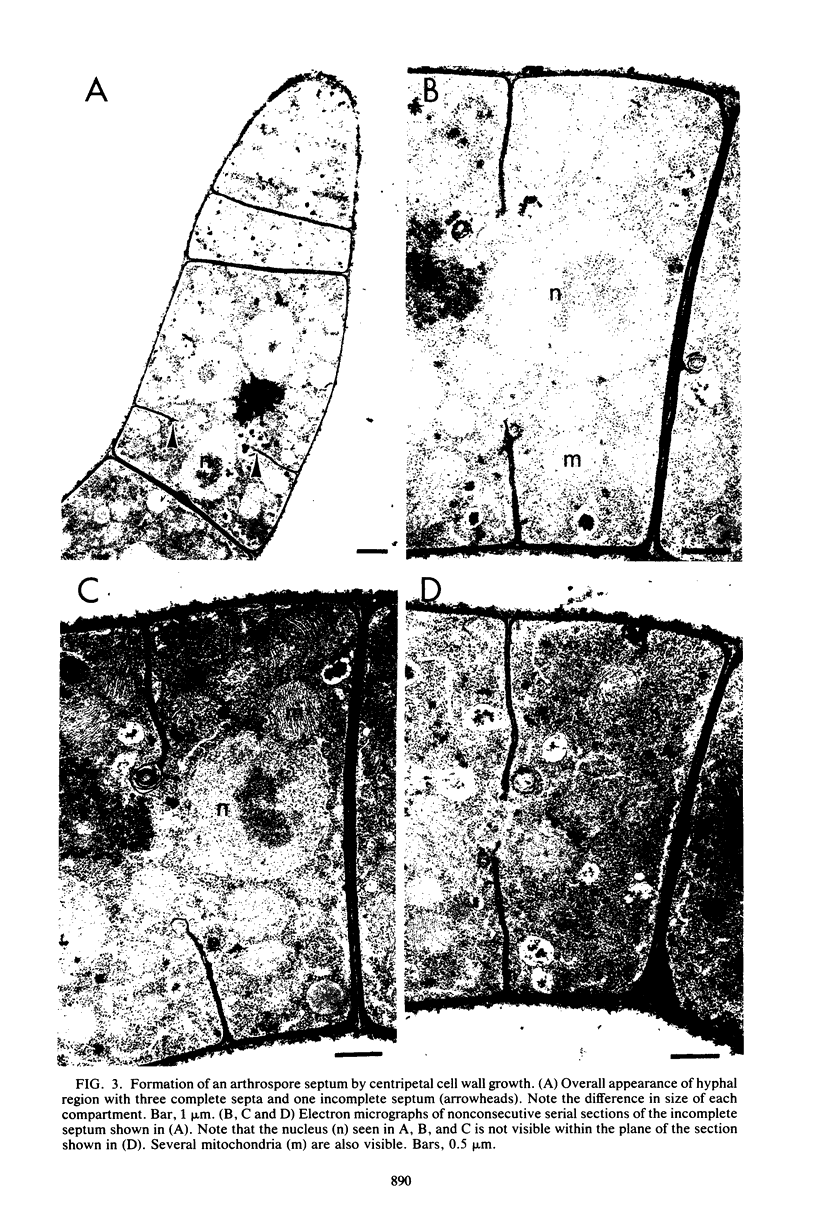
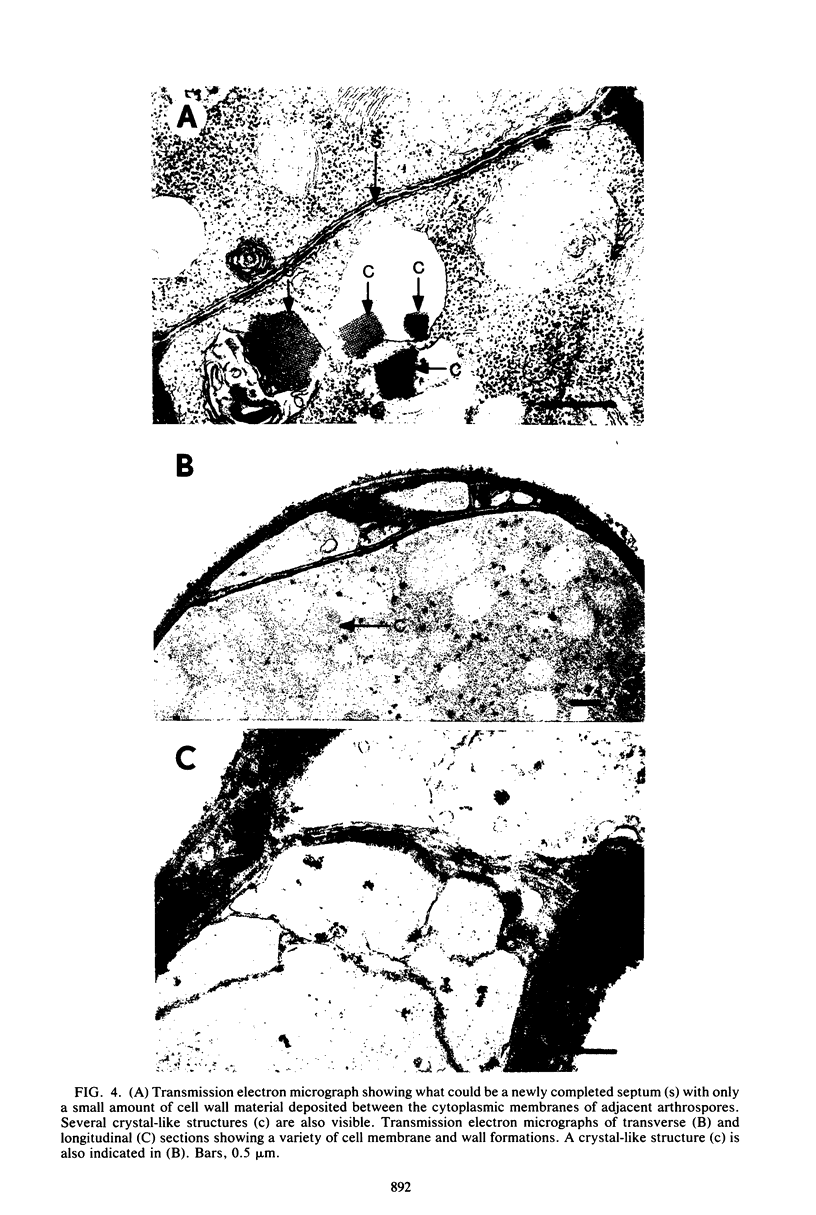
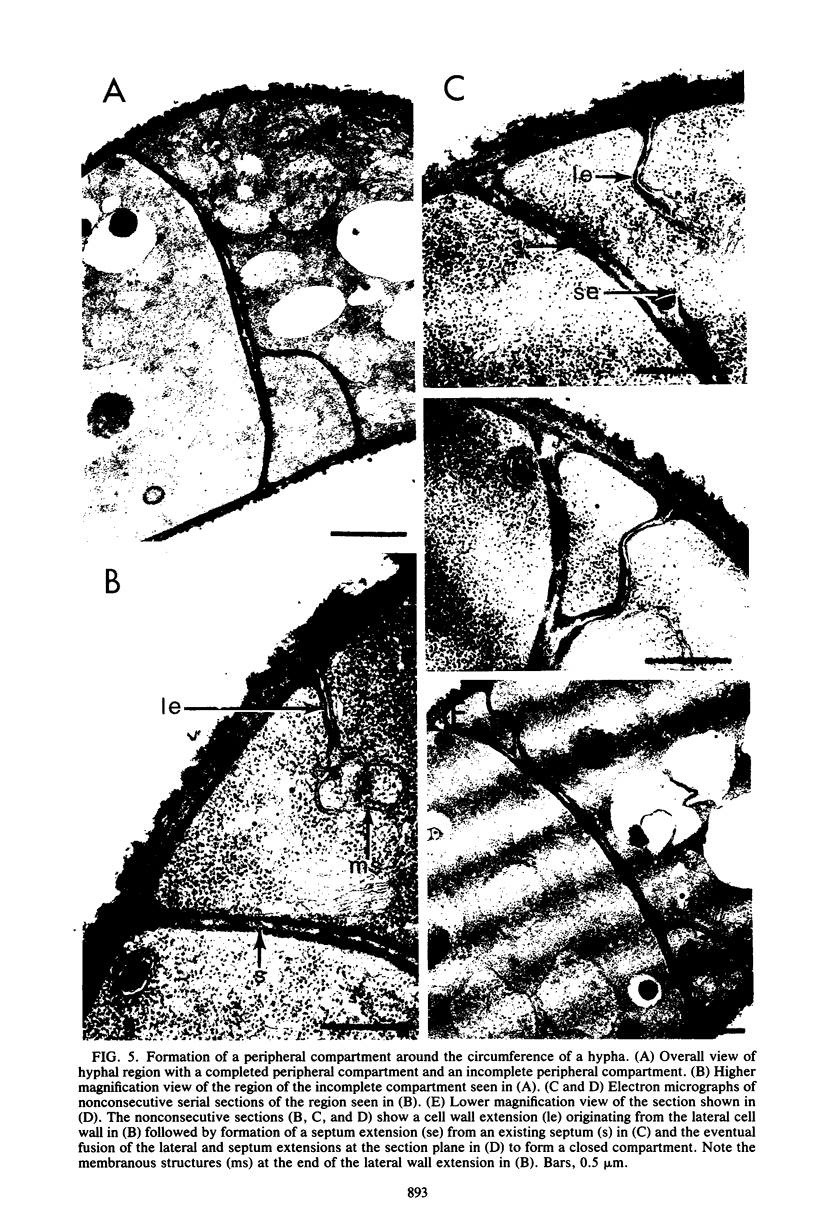
The formation of arthrospores in Mucor rouxii was studied by transmission and scanning electron microscopy and light microscopy. The arthrospores formed in a random manner in terminal and internal regions of the hyphae. The earliest appearance of the arthrospores was seen by scanning electron microscopy as compartments delineated by double ridges. These ridges probably corresponded to the site of septal wall formation. The elongated compartments varied considerably in size. As the arthrospores matured, they tended to separate as a result of a gradual change in the shape of the arthrospores to a nearly spherical form and also as the result of simultaneous degradation of the outermost cell wall layer. The mature arthrospores were surrounded by a complex cell wall consisting of at least three distinct layers in addition to the original hyphal cell wall. Crystal-like structures were seen in the cytoplasm of some of the arthrospores in addition to the usual organelles such as mitochondria, nuclei, and ribosomes. Septum formation by centripetal cell wall growth from the lateral hyphal wall was documented by transmission electron microscopy. However, evidence was also found which suggested that not all internal cell wall development in the fungal hyphae during arthrosporogenesis necessarily led to the formation of mature arthrospores.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BARTNICKI-GARCIA S., NICKERSON W. J. Induction of yeast-like development in Mucor by carbon dioxide. J Bacteriol. 1962 Oct;84:829–840. doi: 10.1128/jb.84.4.829-840.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BARTNICKI-GARCIA S., NICKERSON W. J. Isolation, composition, and structure of cell walls of filamentous and yeast-like forms of Mucor rouxii. Biochim Biophys Acta. 1962 Mar 26;58:102–119. doi: 10.1016/0006-3002(62)90822-3. [DOI] [PubMed] [Google Scholar]
- BARTNICKI-GARCIA S., NICKERSON W. J. Nutrition, growth, and morphogenesis of Mucor rouxii. J Bacteriol. 1962 Oct;84:841–858. doi: 10.1128/jb.84.4.841-858.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bibel D. J., Crumrine D. A., Yee K., King R. D. Development of arthrospores of Trichophyton mentagrophytes. Infect Immun. 1977 Mar;15(3):958–971. doi: 10.1128/iai.15.3.958-971.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dow J. M., Carreon R. R., Villa V. D. Role of membranes of mycelial Mucor rouxii in synthesis and secretion of cell wall matrix polymers. J Bacteriol. 1981 Jan;145(1):272–279. doi: 10.1128/jb.145.1.272-279.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto T., Blumenthal H. J. Factors affecting germination of Trichophyton mentagrophytes arthrospores. Infect Immun. 1977 Nov;18(2):479–486. doi: 10.1128/iai.18.2.479-486.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto T., Blumenthal H. J. Survival and resistance of Trichophyton mentagrophytes arthrospores. Appl Environ Microbiol. 1978 Feb;35(2):274–277. doi: 10.1128/aem.35.2.274-277.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto T., Morgan J., Conti S. F. Morphogenesis and ultrastructure of Geotrichum candidum septa. J Bacteriol. 1973 Oct;116(1):447–455. doi: 10.1128/jb.116.1.447-455.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto T., Pollack J. H., Blumenthal H. J. Carotenogenesis associated with arthrosporulation of Trichophyton mentagrophytes. J Bacteriol. 1978 Dec;136(3):1120–1126. doi: 10.1128/jb.136.3.1120-1126.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley R. O., Dekker R. A., Bluemink J. G. Ligand-mediated osmium binding: its application in coating biological specimens for scanning electron microscopy. J Ultrastruct Res. 1973 Nov;45(3):254–258. doi: 10.1016/s0022-5320(73)80051-6. [DOI] [PubMed] [Google Scholar]
- Künkel W., Hädrich H., Damaschun H., Damaschun G. Alkoholdehydrogenase (ADH) in Hefezellen. III. Strukturuntersuchungen an zellulären ADH-Kristallen von Saccharomyces carlsbergensis mit Hilfe der Elektronenmikroskopie und Röntgenkleinwinkelstreuung. Mikroskopie. 1980 Jun;36(3-4):81–92. [PubMed] [Google Scholar]
- Rippon J. W. Dimorphism in pathogenic fungi. Crit Rev Microbiol. 1980;8(1):49–97. doi: 10.3109/10408418009085078. [DOI] [PubMed] [Google Scholar]
- Spurr A. R. A low-viscosity epoxy resin embedding medium for electron microscopy. J Ultrastruct Res. 1969 Jan;26(1):31–43. doi: 10.1016/s0022-5320(69)90033-1. [DOI] [PubMed] [Google Scholar]
- Wheat R. W., Tritschler C., Conant N. F., Lowe E. P. Comparison of Coccidioides immitis arthrospore, mycelium, and spherule cell walls, and influence of growth medium on mycelial cell wall composition. Infect Immun. 1977 Jul;17(1):91–97. doi: 10.1128/iai.17.1.91-97.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]