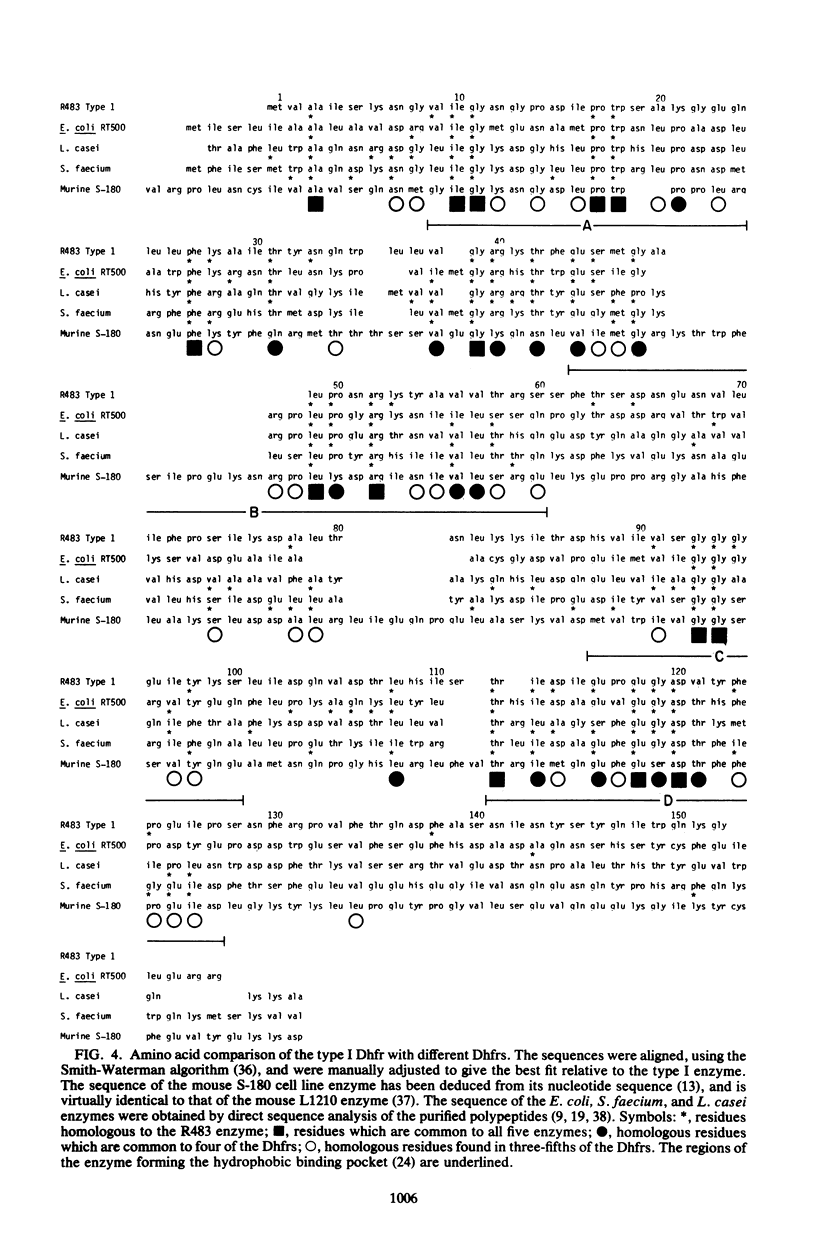
Abstract
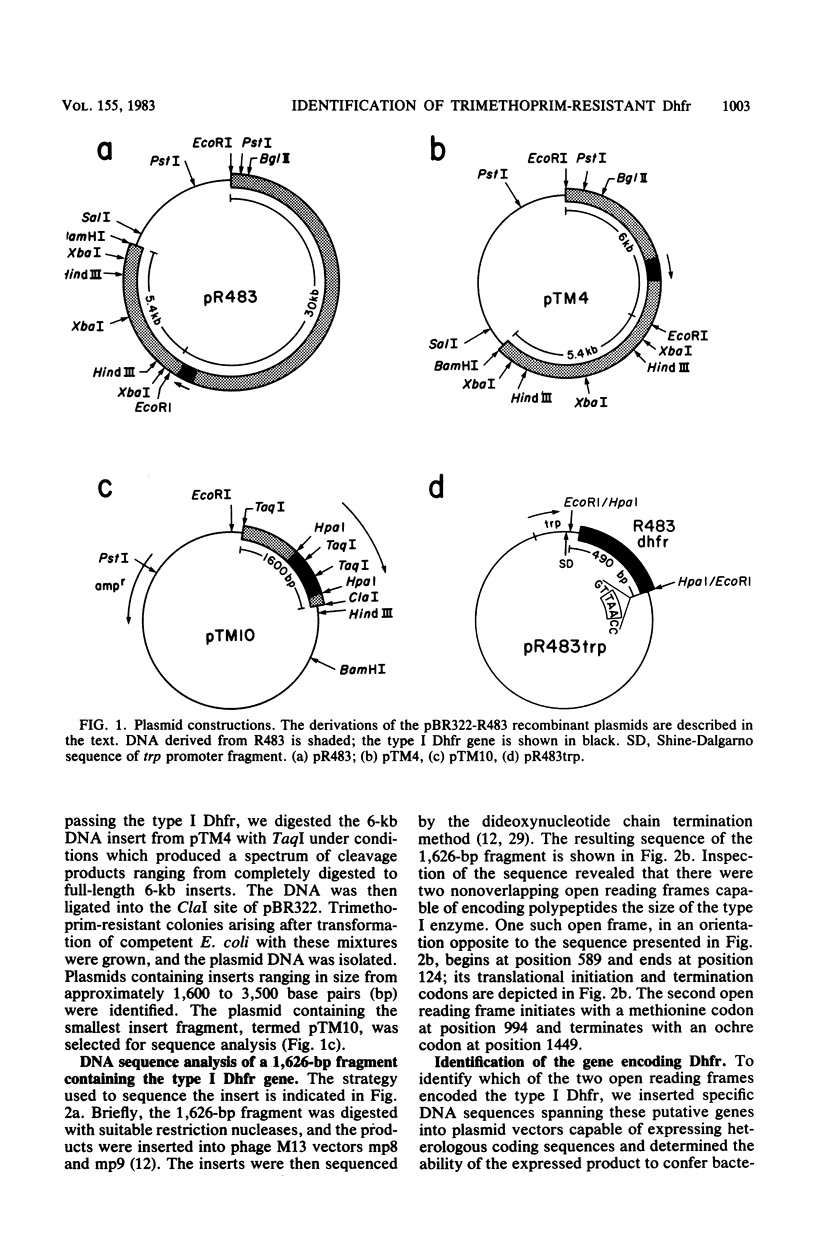
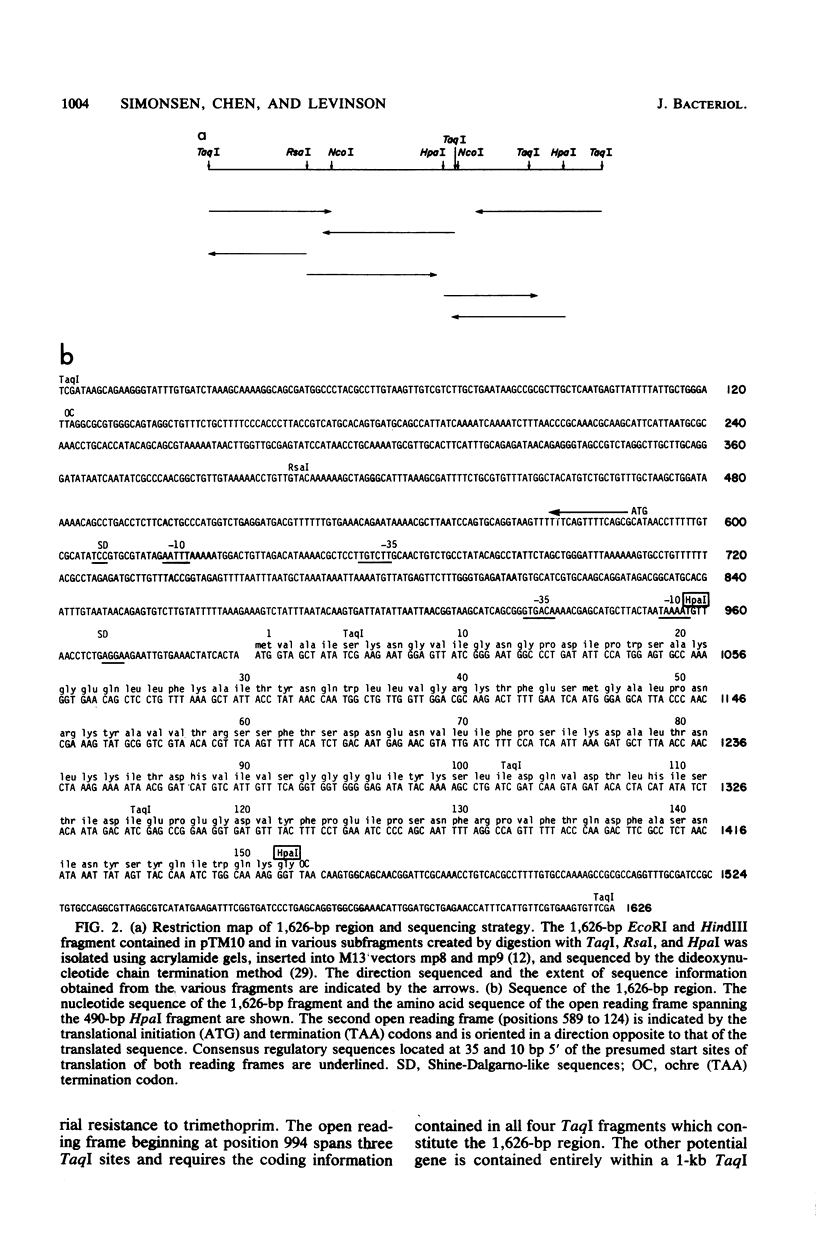
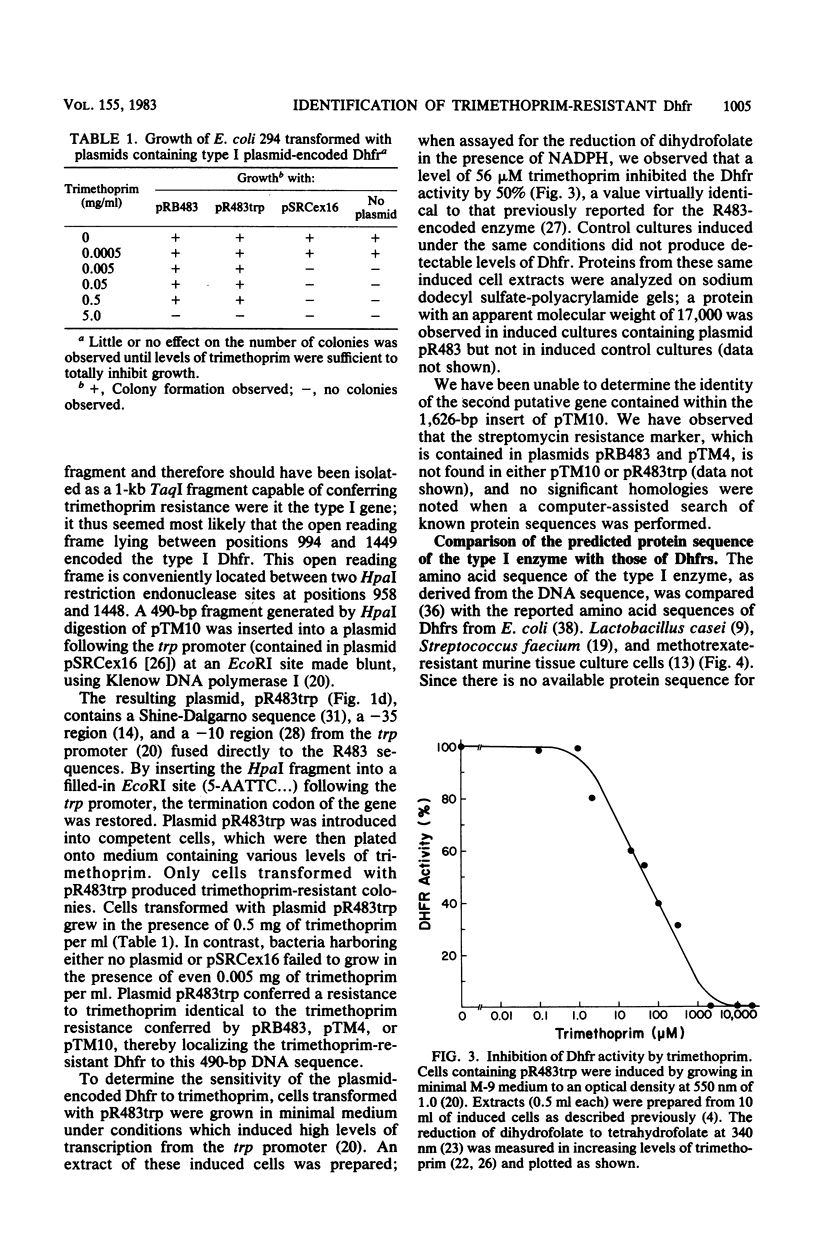
We have isolated and determined the nucleotide sequence of a 1,626-base-pair fragment from R-plasmid R483 which encodes a trimethoprim-resistant dihydrofolate reductase. Analysis of the nucleotide sequence of this fragment revealed the presence of two open reading frames, each sufficient to encode polypeptides of approximately 17,000 daltons. Both open regions are preceded by sequences conforming closely to the canonical description of procaryotic promoters. A 490-base-pair HpaI fragment spanning one of the potential coding regions was inserted into a plasmid vector under the transcriptional control of the trp promoter. Cells transformed with this plasmid were trimethoprim resistant and produced dihydrofolate reductase activity which in vitro was resistant to moderate levels of trimethoprim. Analysis of the predicted amino acid sequence of this protein indicated that the R483-encoded trimethoprim-resistant enzyme was distantly related to the trimethoprim-sensitive bacterial homologs. The conserved amino acids were localized primarily to the region of the enzyme previously shown to comprise the hydrophobic substrate binding pocket.
Full text
PDF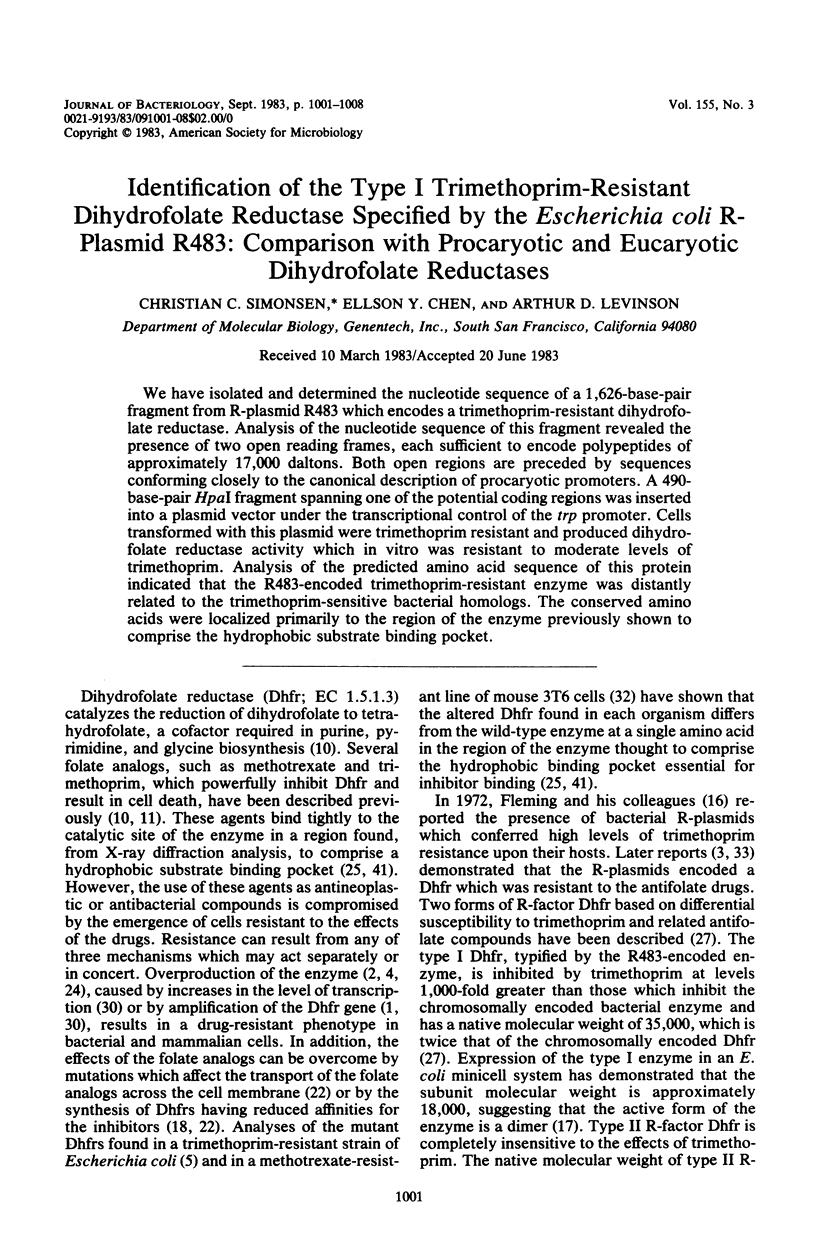



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alt F. W., Kellems R. E., Bertino J. R., Schimke R. T. Selective multiplication of dihydrofolate reductase genes in methotrexate-resistant variants of cultured murine cells. J Biol Chem. 1978 Mar 10;253(5):1357–1370. [PubMed] [Google Scholar]
- Alt F. W., Kellems R. E., Schimke R. T. Synthesis and degradation of folate reductase in sensitive and methotrexate-resistant lines of S-180 cells. J Biol Chem. 1976 May 25;251(10):3063–3074. [PubMed] [Google Scholar]
- Amyes S. G., Smith J. T. R-factor trimethoprim resistance mechanism: an insusceptible target site. Biochem Biophys Res Commun. 1974 May 20;58(2):412–418. doi: 10.1016/0006-291x(74)90380-5. [DOI] [PubMed] [Google Scholar]
- Baccanari D. P., Stone D., Kuyper L. Effect of a single amino acid substitution on Escherichia coli dihydrofolate reductase catalysis and ligand binding. J Biol Chem. 1981 Feb 25;256(4):1738–1747. [PubMed] [Google Scholar]
- Baccanari D., Phillips A., Smith S., Sinski D., Burchall J. Purification and properties of Escherichia coli dihydrofolate reductase. Biochemistry. 1975 Dec 2;14(24):5267–5273. doi: 10.1021/bi00695a006. [DOI] [PubMed] [Google Scholar]
- Backman K., Ptashne M., Gilbert W. Construction of plasmids carrying the cI gene of bacteriophage lambda. Proc Natl Acad Sci U S A. 1976 Nov;73(11):4174–4178. doi: 10.1073/pnas.73.11.4174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barth P. T., Datta N., Hedges R. W., Grinter N. J. Transposition of a deoxyribonucleic acid sequence encoding trimethoprim and streptomycin resistances from R483 to other replicons. J Bacteriol. 1976 Mar;125(3):800–810. doi: 10.1128/jb.125.3.800-810.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bitar K. G., Blankenship D. T., Walsh K. A., Dunlap R. B., Reddy A. V., Freisheim J. H. Amino acid sequence of dihydrofolate reductase from an amethopterin-resistant strain of Lactobacillus casei. FEBS Lett. 1977 Aug 1;80(1):119–122. doi: 10.1016/0014-5793(77)80420-1. [DOI] [PubMed] [Google Scholar]
- Burchall J. J., Hitchings G. H. Inhibitor binding analysis of dihydrofolate reductases from various species. Mol Pharmacol. 1965 Sep;1(2):126–136. [PubMed] [Google Scholar]
- Chen E. Y., Howley P. M., Levinson A. D., Seeburg P. H. The primary structure and genetic organization of the bovine papillomavirus type 1 genome. Nature. 1982 Oct 7;299(5883):529–534. doi: 10.1038/299529a0. [DOI] [PubMed] [Google Scholar]
- Crouse G. F., Simonsen C. C., McEwan R. N., Schimke R. T. Structure of amplified normal and variant dihydrofolate reductase genes in mouse sarcoma S180 cells. J Biol Chem. 1982 Jul 10;257(13):7887–7897. [PubMed] [Google Scholar]
- Doolittle R. F. Similar amino acid sequences: chance or common ancestry? Science. 1981 Oct 9;214(4517):149–159. doi: 10.1126/science.7280687. [DOI] [PubMed] [Google Scholar]
- Fleming M. P., Datta N., Grüneberg R. N. Trimethoprim resistance determined by R factors. Br Med J. 1972 Mar 18;1(5802):726–728. doi: 10.1136/bmj.1.5802.726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fling M. E., Elwell L. P. Protein expression in Escherichia coli minicells containing recombinant plasmids specifying trimethoprim-resistant dihydrofolate reductases. J Bacteriol. 1980 Feb;141(2):779–785. doi: 10.1128/jb.141.2.779-785.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flintoff W. F., Davidson S. V., Siminovitch L. Isolation and partial characterization of three methotrexate-resistant phenotypes from Chinese hamster ovary cells. Somatic Cell Genet. 1976 May;2(3):245–261. doi: 10.1007/BF01538963. [DOI] [PubMed] [Google Scholar]
- Gleisner J. M., Peterson D. L., Blakley R. L. Amino-acid sequence of dihydrofolate reductase from a methotrexate-resistant mutant of Streptococcus faecium and identification of methionine residues at the inhibitor binding site. Proc Natl Acad Sci U S A. 1974 Aug;71(8):3001–3005. doi: 10.1073/pnas.71.8.3001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goeddel D. V., Heyneker H. L., Hozumi T., Arentzen R., Itakura K., Yansura D. G., Ross M. J., Miozzari G., Crea R., Seeburg P. H. Direct expression in Escherichia coli of a DNA sequence coding for human growth hormone. Nature. 1979 Oct 18;281(5732):544–548. doi: 10.1038/281544a0. [DOI] [PubMed] [Google Scholar]
- Gray P. W., Leung D. W., Pennica D., Yelverton E., Najarian R., Simonsen C. C., Derynck R., Sherwood P. J., Wallace D. M., Berger S. L. Expression of human immune interferon cDNA in E. coli and monkey cells. Nature. 1982 Feb 11;295(5849):503–508. doi: 10.1038/295503a0. [DOI] [PubMed] [Google Scholar]
- Haber D. A., Schimke R. T. Unstable amplification of an altered dihydrofolate reductase gene associated with double-minute chromosomes. Cell. 1981 Nov;26(3 Pt 1):355–362. doi: 10.1016/0092-8674(81)90204-x. [DOI] [PubMed] [Google Scholar]
- Hillcoat B. L., Blakley R. L. Dihydrofolate reductase of Streptococcus faecalis. I. Purification and some properties of reductase from the wild strain and from strain A. J Biol Chem. 1966 Jul 10;241(13):2995–3001. [PubMed] [Google Scholar]
- Kellems R. E., Alt F. W., Schimke R. T. Regulation of folate reductase synthesis in sensitive and methotrexate-resistant sarcoma 180 cells. In vitro translation and characterization of folate reductase mRNA. J Biol Chem. 1976 Nov 25;251(22):6987–6993. [PubMed] [Google Scholar]
- Matthews D. A., Alden R. A., Bolin J. T., Freer S. T., Hamlin R., Xuong N., Kraut J., Poe M., Williams M., Hoogsteen K. Dihydrofolate reductase: x-ray structure of the binary complex with methotrexate. Science. 1977 Jul 29;197(4302):452–455. doi: 10.1126/science.17920. [DOI] [PubMed] [Google Scholar]
- McGrath J. P., Levinson A. D. Bacterial expression of an enzymatically active protein encoded by RSV src gene. Nature. 1982 Feb 4;295(5848):423–425. doi: 10.1038/295423a0. [DOI] [PubMed] [Google Scholar]
- Pattishall K. H., Acar J., Burchall J. J., Goldstein F. W., Harvey R. J. Two distinct types of trimethoprim-resistant dihydrofolate reductase specified by R-plasmids of different compatibility groups. J Biol Chem. 1977 Apr 10;252(7):2319–2323. [PubMed] [Google Scholar]
- Pribnow D. Bacteriophage T7 early promoters: nucleotide sequences of two RNA polymerase binding sites. J Mol Biol. 1975 Dec 15;99(3):419–443. doi: 10.1016/s0022-2836(75)80136-7. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheldon R., Brenner S. Regulatory mutants of dihydrofolate reductase in Escherichia coli K12. Mol Gen Genet. 1976 Aug 10;147(1):91–97. doi: 10.1007/BF00337941. [DOI] [PubMed] [Google Scholar]
- Shine J., Dalgarno L. The 3'-terminal sequence of Escherichia coli 16S ribosomal RNA: complementarity to nonsense triplets and ribosome binding sites. Proc Natl Acad Sci U S A. 1974 Apr;71(4):1342–1346. doi: 10.1073/pnas.71.4.1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonsen C. C., Levinson A. D. Isolation and expression of an altered mouse dihydrofolate reductase cDNA. Proc Natl Acad Sci U S A. 1983 May;80(9):2495–2499. doi: 10.1073/pnas.80.9.2495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sköld O., Widh A. A new dihydrofolate reductase with low trimethoprim sensitivity induced by an R factor mediating high resistance to trimethoprim. J Biol Chem. 1974 Jul 10;249(13):4324–4325. [PubMed] [Google Scholar]
- Smith D. R., Calvo J. M. Nucleotide sequence of the E coli gene coding for dihydrofolate reductase. Nucleic Acids Res. 1980 May 24;8(10):2255–2274. doi: 10.1093/nar/8.10.2255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith S. L., Stone D., Novak P., Baccanari D. P., Burchall J. J. R plasmid dihydrofolate reductase with subunit structure. J Biol Chem. 1979 Jul 25;254(14):6222–6225. [PubMed] [Google Scholar]
- Smith T. F., Waterman M. S. Identification of common molecular subsequences. J Mol Biol. 1981 Mar 25;147(1):195–197. doi: 10.1016/0022-2836(81)90087-5. [DOI] [PubMed] [Google Scholar]
- Stone D., Paterson S. J., Raper J. H., Phillips A. W. The amino acid sequence of dihydrofolate reductase from the mouse lymphoma L1210. J Biol Chem. 1979 Jan 25;254(2):480–488. [PubMed] [Google Scholar]
- Stone D., Phillips A. W., Burchall J. J. The amino-acid sequence of the dihydrofolate reductase of a trimethoprim-resistant strain of Escherichia coli. Eur J Biochem. 1977 Feb;72(3):613–624. doi: 10.1111/j.1432-1033.1977.tb11284.x. [DOI] [PubMed] [Google Scholar]
- Stone D., Smith S. L. The amino acid sequence of the trimethoprim-resistant dihydrofolate reductase specified in Escherichia coli by R-plasmid R67. J Biol Chem. 1979 Nov 10;254(21):10857–10861. [PubMed] [Google Scholar]
- Swift G., McCarthy B. J., Heffron F. DNA sequence of a plasmid-encoded dihydrofolate reductase. Mol Gen Genet. 1981;181(4):441–447. doi: 10.1007/BF00428733. [DOI] [PubMed] [Google Scholar]
- Volz K. W., Matthews D. A., Alden R. A., Freer S. T., Hansch C., Kaufman B. T., Kraut J. Crystal structure of avian dihydrofolate reductase containing phenyltriazine and NADPH. J Biol Chem. 1982 Mar 10;257(5):2528–2536. [PubMed] [Google Scholar]


