Abstract
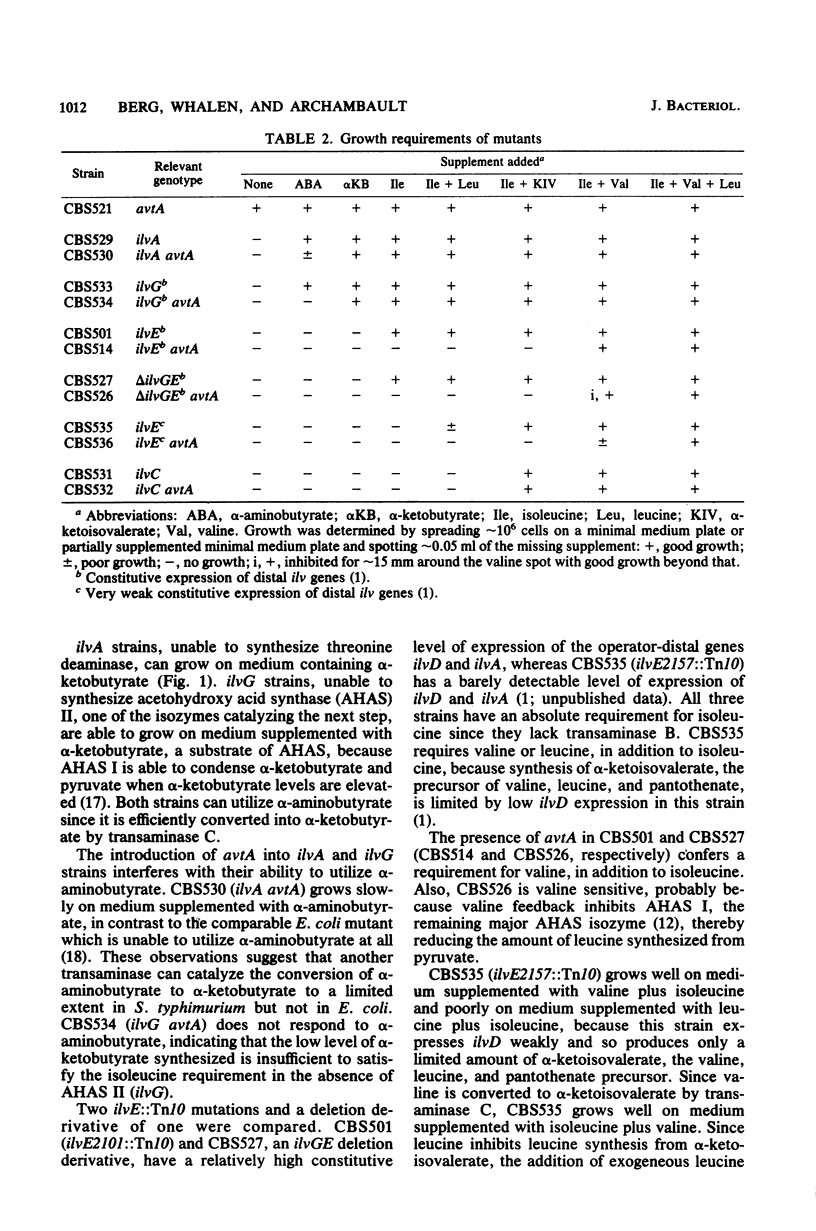
In Salmonella typhimurium, as in Escherichia coli, mutations in avtA, the gene encoding the alanine-valine transaminase (transaminase C), are silent unless they are combined with mutations involved in isoleucine-valine biosynthesis. avtA is repressed by leucine or alanine but not by valine. Transaminase C is found at reduced levels upon starvation for any one of several amino acids. We hypothesize that this is due to repression of avtA by the elevated alanine and leucine pools found in amino acid-starved cells.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
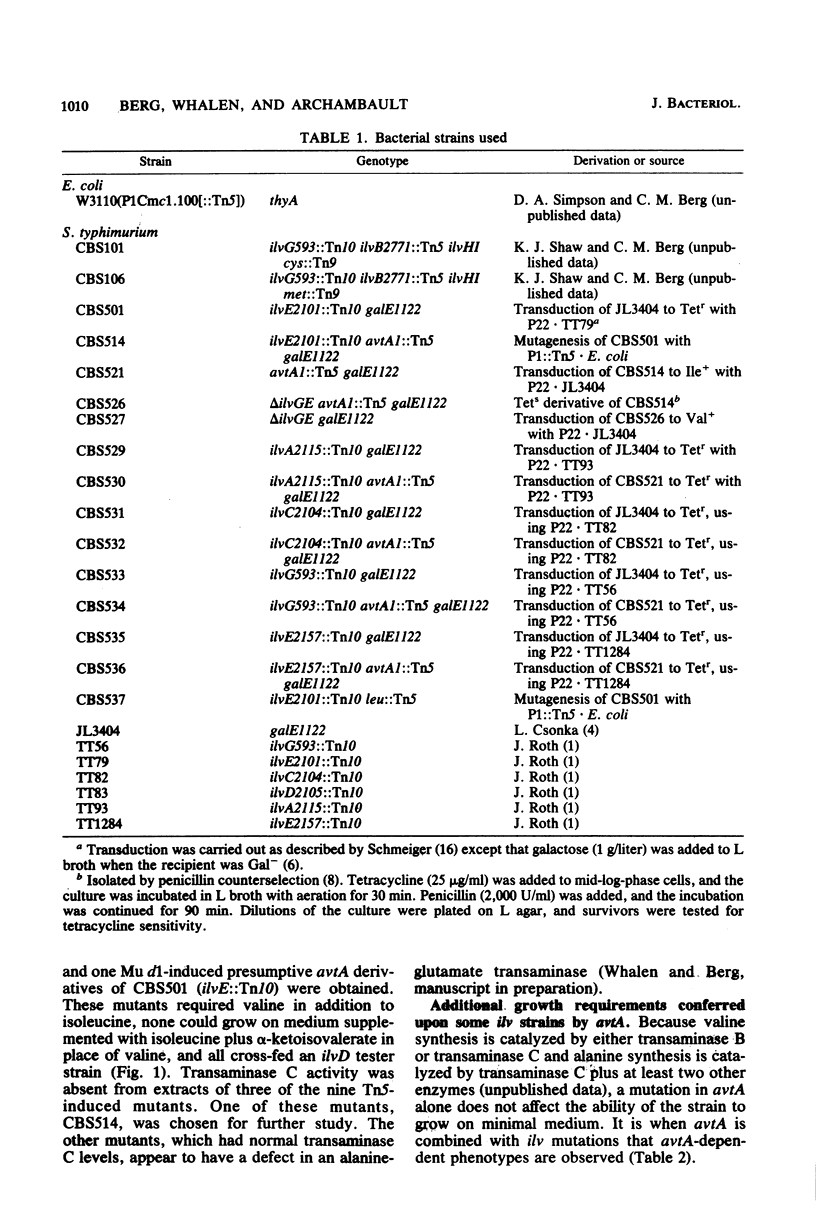
- Berg C. M., Shaw K. J. Organization and regulation of the ilvGEDA operon in Salmonella typhimurium LT2. J Bacteriol. 1981 Feb;145(2):984–989. doi: 10.1128/jb.145.2.984-989.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg C. M., Shaw K. J., Vender J., Borucka-Mankiewicz M. Physiological characterization of polar Tn5-induced isoleucine-valine auxotrophs in Escherichia coli K.12: evidence for an internal promoter in the ilvOGEDA operon. Genetics. 1979 Oct;93(2):308–319. [PMC free article] [PubMed] [Google Scholar]
- Casadaban M. J., Cohen S. N. Lactose genes fused to exogenous promoters in one step using a Mu-lac bacteriophage: in vivo probe for transcriptional control sequences. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4530–4533. doi: 10.1073/pnas.76.9.4530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtiss R., 3rd, Charamella L. J., Berg C. M., Harris P. E. Kinetic and genetic analyses of D-cycloserine inhibition and resistance in Escherichia coli. J Bacteriol. 1965 Nov;90(5):1238–1250. doi: 10.1128/jb.90.5.1238-1250.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enomoto M., Stocker B. A. Transduction by phage P1kc in Salmonella typhimurium. Virology. 1974 Aug;60(2):503–514. doi: 10.1016/0042-6822(74)90344-4. [DOI] [PubMed] [Google Scholar]
- Gelfand D. H., Steinberg R. A. Escherichia coli mutants deficient in the aspartate and aromatic amino acid aminotransferases. J Bacteriol. 1977 Apr;130(1):429–440. doi: 10.1128/jb.130.1.429-440.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
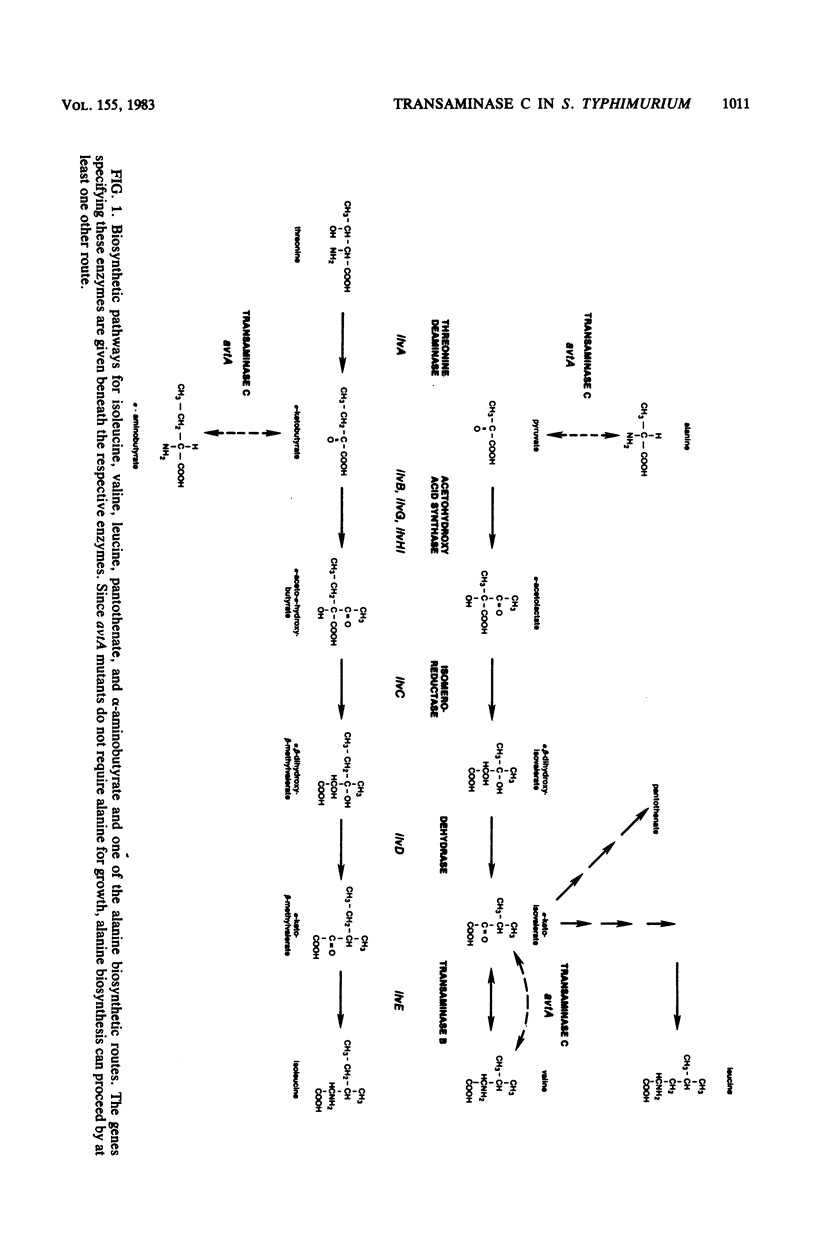
- Jensen R. A., Calhoun D. H. Intracellular roles of microbial aminotransferases: overlap enzymes across different biochemical pathways. Crit Rev Microbiol. 1981;8(3):229–266. doi: 10.3109/10408418109085080. [DOI] [PubMed] [Google Scholar]
- McGilvray D., Umbarger H. E. Regulation of transaminase C synthesis in Escherichia coli: conditional leucine auxotrophy. J Bacteriol. 1974 Nov;120(2):715–723. doi: 10.1128/jb.120.2.715-723.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Neill J. P., Freundlich M. Temperature-sensitive growth inhibition by valine in Salmonella typhimurium: alteration of one form of acetohydroxy acid synthetase. J Bacteriol. 1973 Oct;116(1):98–106. doi: 10.1128/jb.116.1.98-106.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RUDMAN D., MEISTER A. Transamination in Escherichia coli. J Biol Chem. 1953 Feb;200(2):591–604. [PubMed] [Google Scholar]
- Sanderson K. E., Hartman P. E. Linkage map of Salmonella typhimurium, edition V. Microbiol Rev. 1978 Jun;42(2):471–519. doi: 10.1128/mr.42.2.471-519.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw K. J., Berg C. M., Sobol T. J. Salmonella typhimurium mutants defective in acetohydroxy acid synthases I and II. J Bacteriol. 1980 Mar;141(3):1258–1263. doi: 10.1128/jb.141.3.1258-1263.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whalen W. A., Berg C. M. Analysis of an avtA::Mu d1(Ap lac) mutant: metabolic role of transaminase C. J Bacteriol. 1982 May;150(2):739–746. doi: 10.1128/jb.150.2.739-746.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]


