Abstract
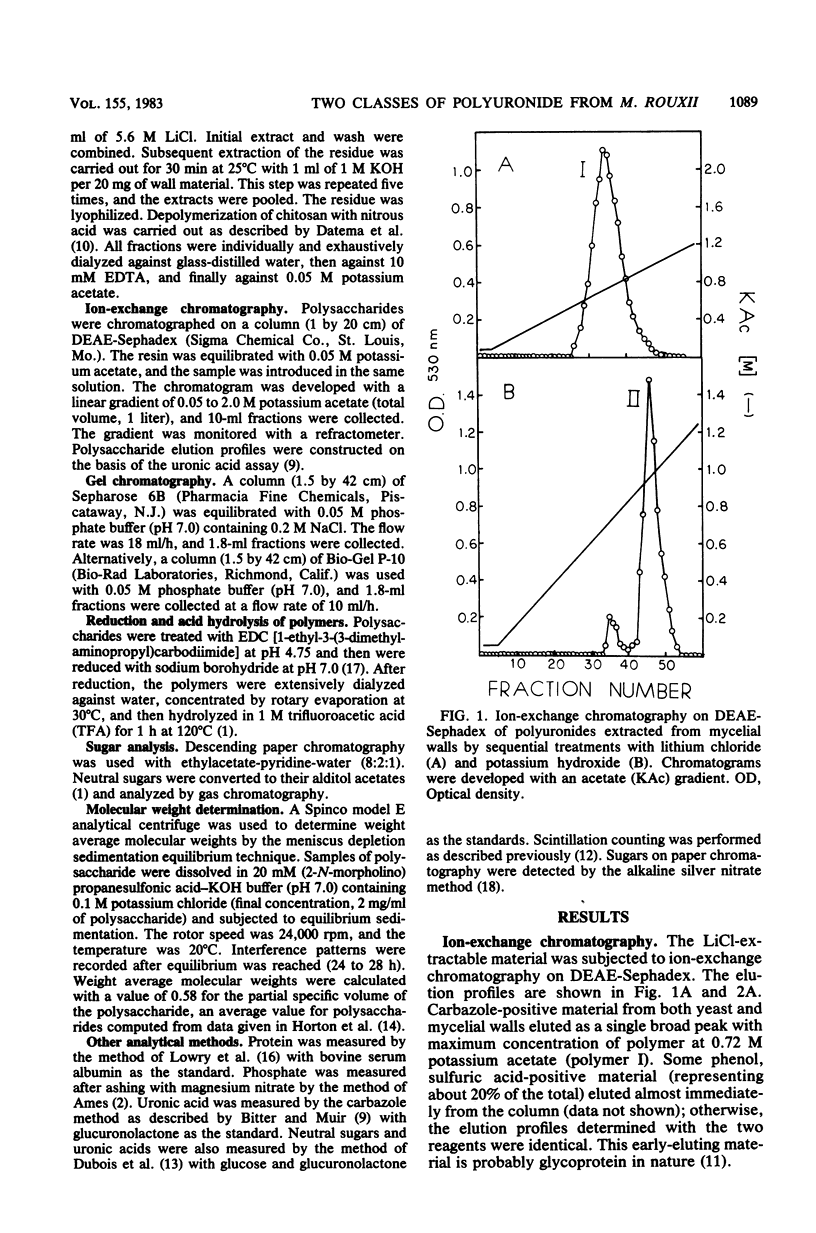
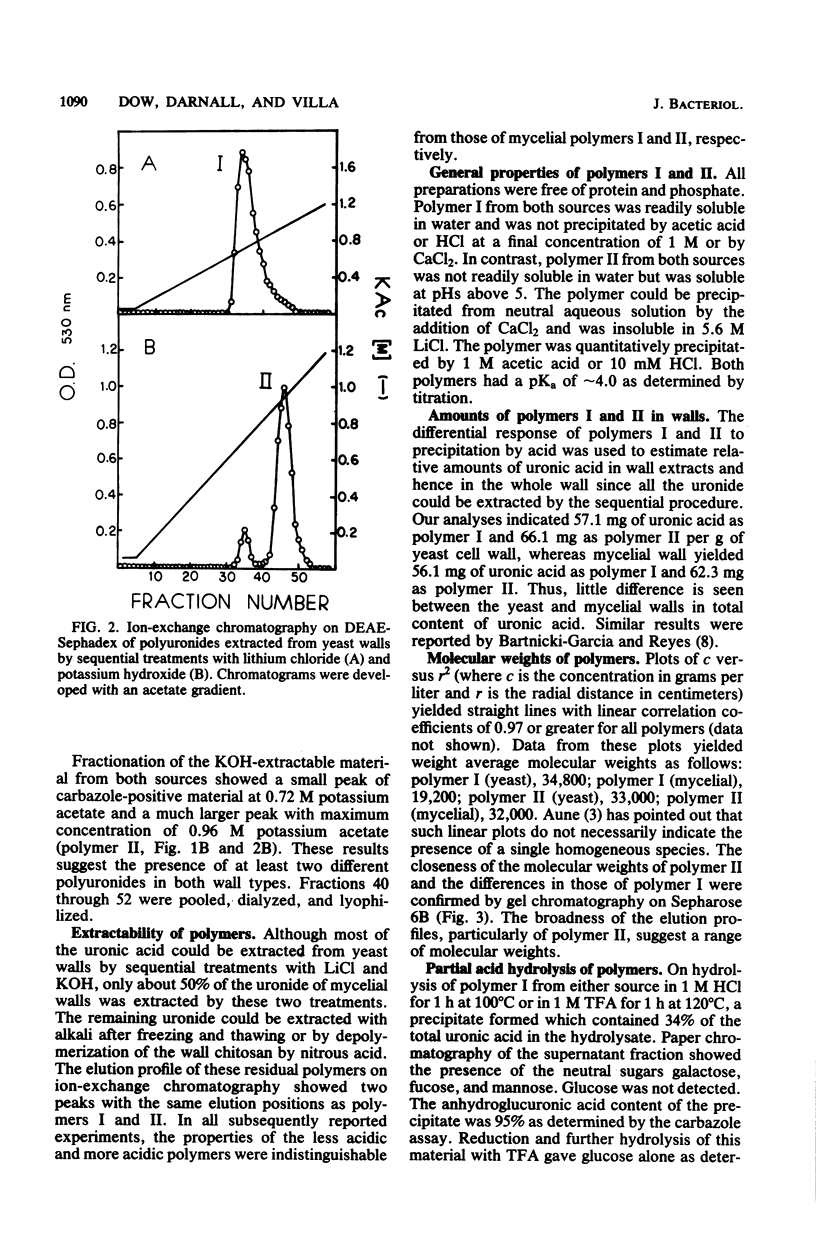
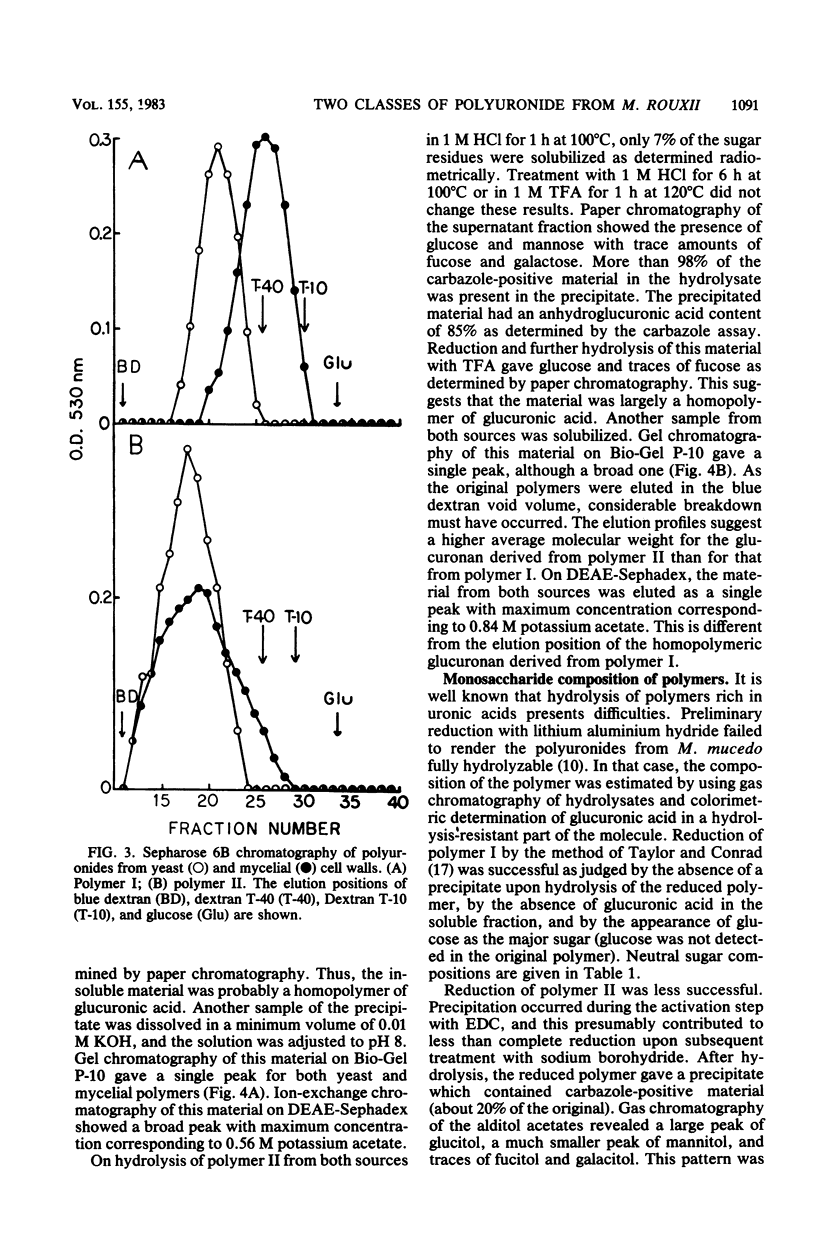
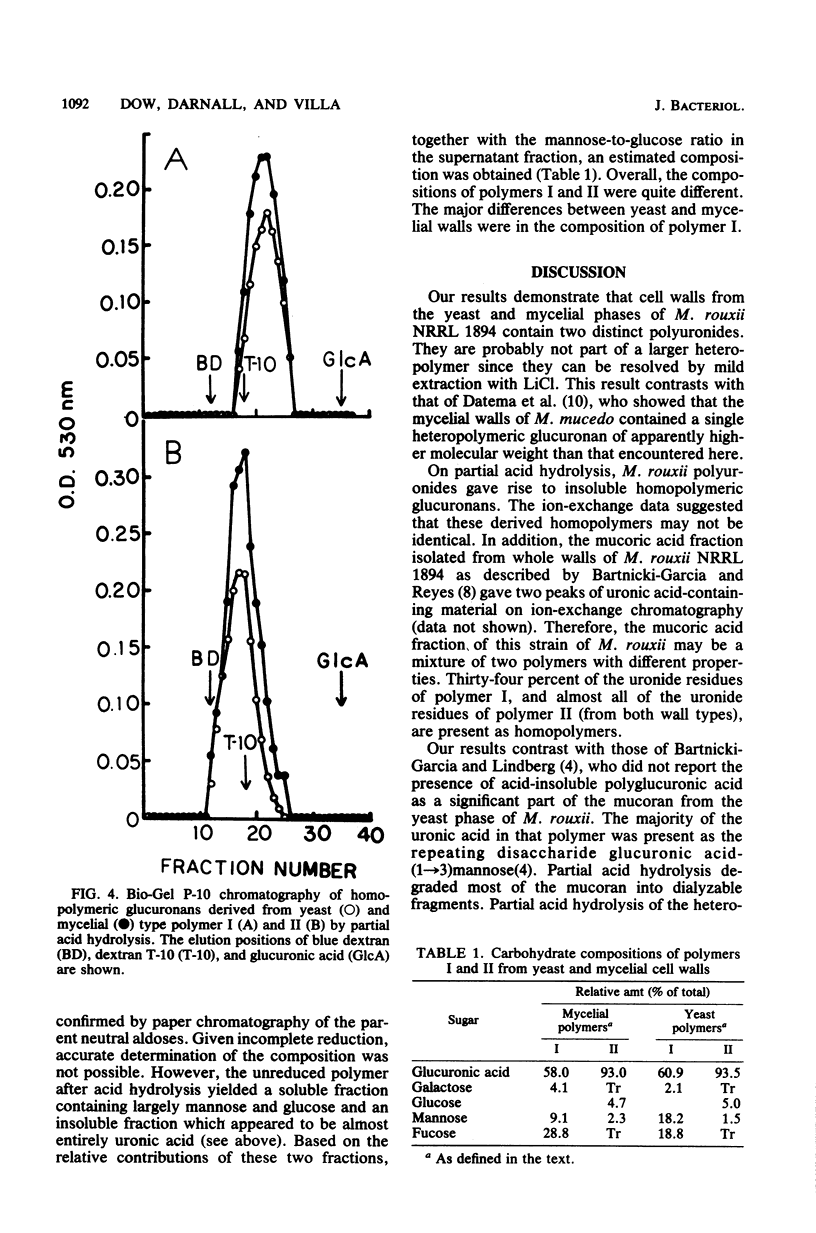
Polyuronides were extracted from purified yeast and mycelial walls of Mucor rouxii by sequential treatments with lithium chloride and potassium hydroxide and were fractionated by ion-exchange chromatography on DEAE-Sephadex. Two polymers (I and II) of different acidity were found in both wall types. Polymer I contained D-glucuronic acid, L-fucose, D-mannose, and much smaller amounts of D-galactose. Yeast and mycelial polymer I had similar uronic acid contents but differed in their neutral sugar compositions and molecular weights. Polymer II from both cell types contained largely D-glucuronic acid and had similar molecular weights. On partial acid hydrolysis, both polymers I and II gave rise to insoluble glucuronans which appeared to be homopolymeric. One-third of the total uronosyl residues of polymer I, and almost all of the uronosyl residues of polymer II, were present in homopolymeric segments. However, homopolymers derived from polymers I and II may not be identical.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aune K. C. Molecular weight measurements by sedimentation equilibrium: some common pitfalls and how to avoid them. Methods Enzymol. 1978;48:163–185. doi: 10.1016/s0076-6879(78)48009-7. [DOI] [PubMed] [Google Scholar]
- BARTNICKI-GARCIA S., NICKERSON W. J. Isolation, composition, and structure of cell walls of filamentous and yeast-like forms of Mucor rouxii. Biochim Biophys Acta. 1962 Mar 26;58:102–119. doi: 10.1016/0006-3002(62)90822-3. [DOI] [PubMed] [Google Scholar]
- BARTNICKI-GARCIA S., NICKERSON W. J. Nutrition, growth, and morphogenesis of Mucor rouxii. J Bacteriol. 1962 Oct;84:841–858. doi: 10.1128/jb.84.4.841-858.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BITTER T., MUIR H. M. A modified uronic acid carbazole reaction. Anal Biochem. 1962 Oct;4:330–334. doi: 10.1016/0003-2697(62)90095-7. [DOI] [PubMed] [Google Scholar]
- Bartnicki-Garcia S., Lindberg B. Partial characterization of mucoran: the glucuronomannan component. Carbohydr Res. 1972 Jun;23(1):75–85. doi: 10.1016/s0008-6215(00)81579-7. [DOI] [PubMed] [Google Scholar]
- Bartnicki-Garcia S., Lippman E. Fungal morphogenesis: cell wall construction in Mucor rouxii. Science. 1969 Jul 18;165(3890):302–304. doi: 10.1126/science.165.3890.302. [DOI] [PubMed] [Google Scholar]
- Bartnicki-Garcia S., Reyes E. Polyuronides in the cell walls of Mucor rouxii. Biochim Biophys Acta. 1968 Nov 12;170(1):54–62. doi: 10.1016/0304-4165(68)90160-8. [DOI] [PubMed] [Google Scholar]
- Datema R., van den Ende H., Wessels J. G. The hyphal wall of Mucor mucedo. 1. Polyanionic polymers. Eur J Biochem. 1977 Nov 1;80(2):611–619. doi: 10.1111/j.1432-1033.1977.tb11918.x. [DOI] [PubMed] [Google Scholar]
- Dow J. M., Villa V. D. Oligoglucuronide production in Mucor rouxii: evidence for a role for endohydrolases in hyphal extension. J Bacteriol. 1980 Jun;142(3):939–944. doi: 10.1128/jb.142.3.939-944.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreger D. R. Polyuronides as structural components of cell walls of fungi and green algae. Nature. 1970 Jul 4;227(5253):81–82. doi: 10.1038/227081a0. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- TREVELYAN W. E., PROCTER D. P., HARRISON J. S. Detection of sugars on paper chromatograms. Nature. 1950 Sep 9;166(4219):444–445. doi: 10.1038/166444b0. [DOI] [PubMed] [Google Scholar]
- Taylor R. L., Conrad H. E. Stoichiometric depolymerization of polyuronides and glycosaminoglycuronans to monosaccharides following reduction of their carbodiimide-activated carboxyl groups. Biochemistry. 1972 Apr 11;11(8):1383–1388. doi: 10.1021/bi00758a009. [DOI] [PubMed] [Google Scholar]


