Abstract
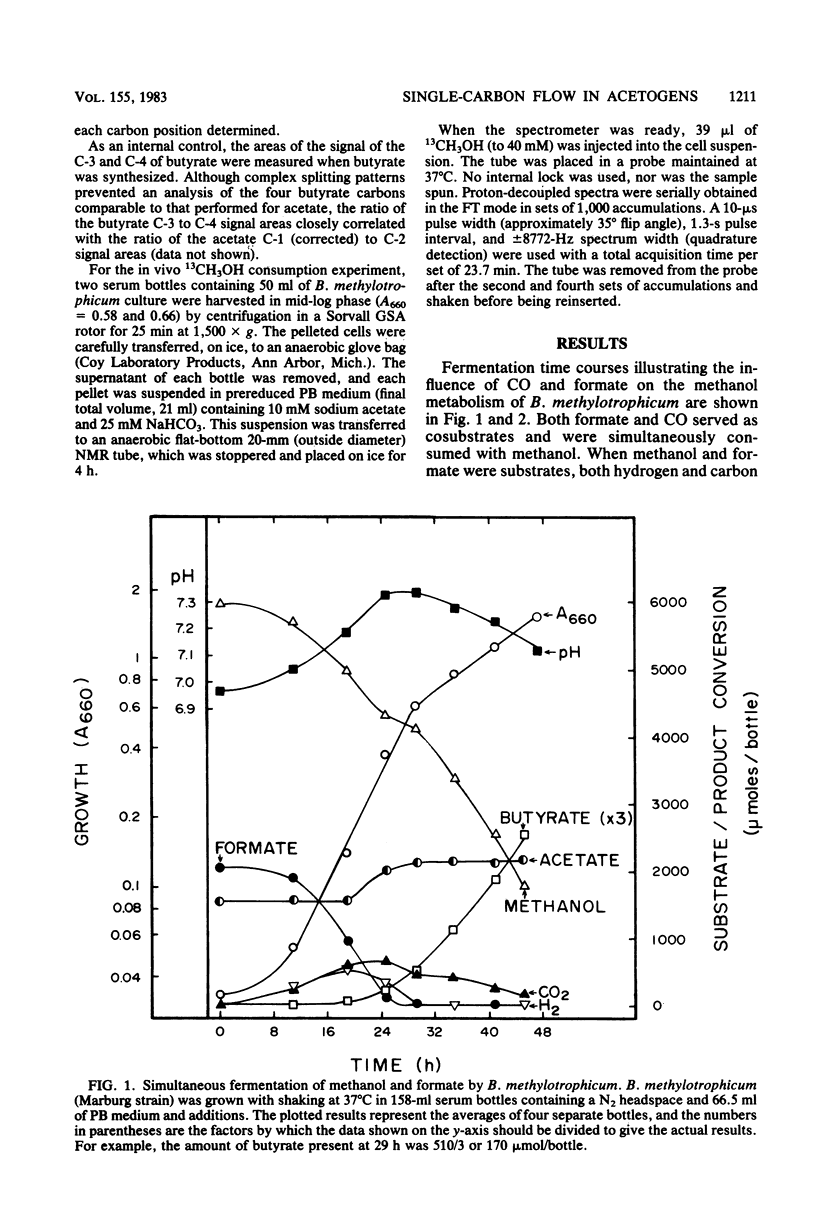
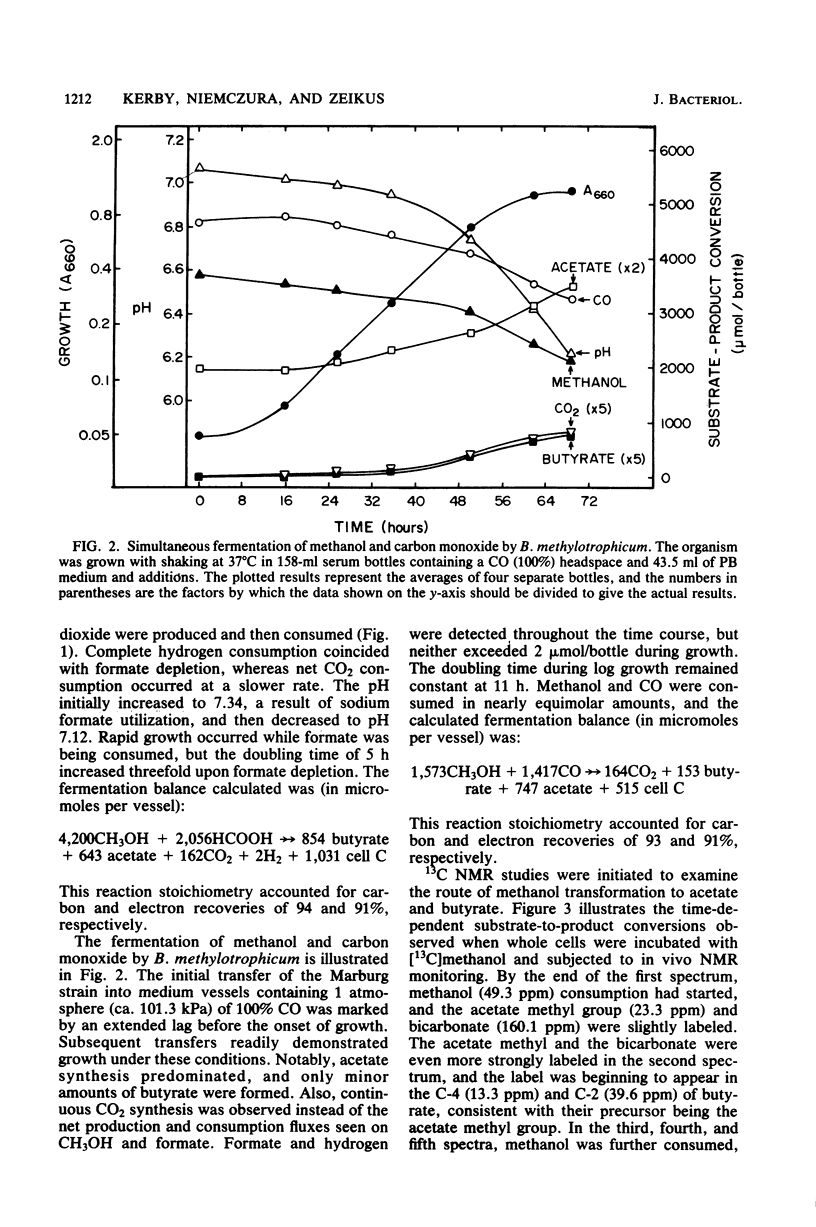
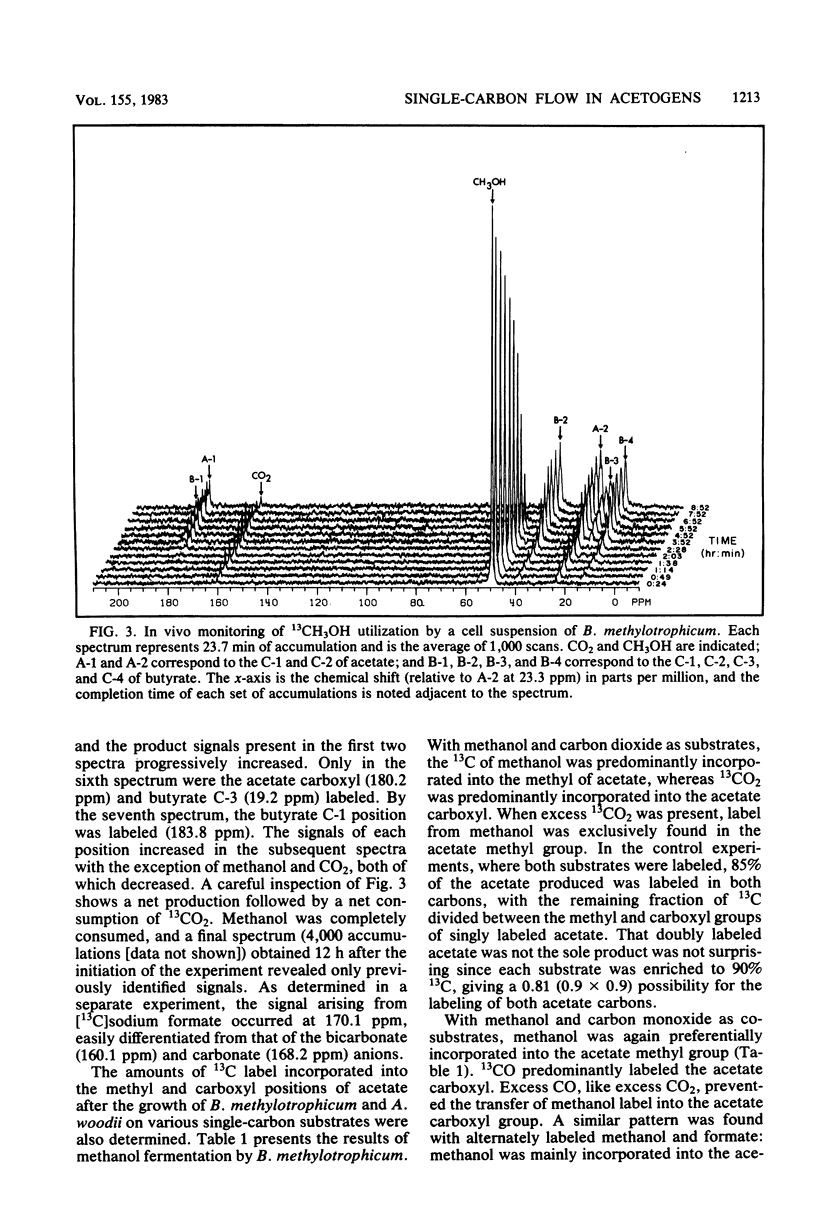
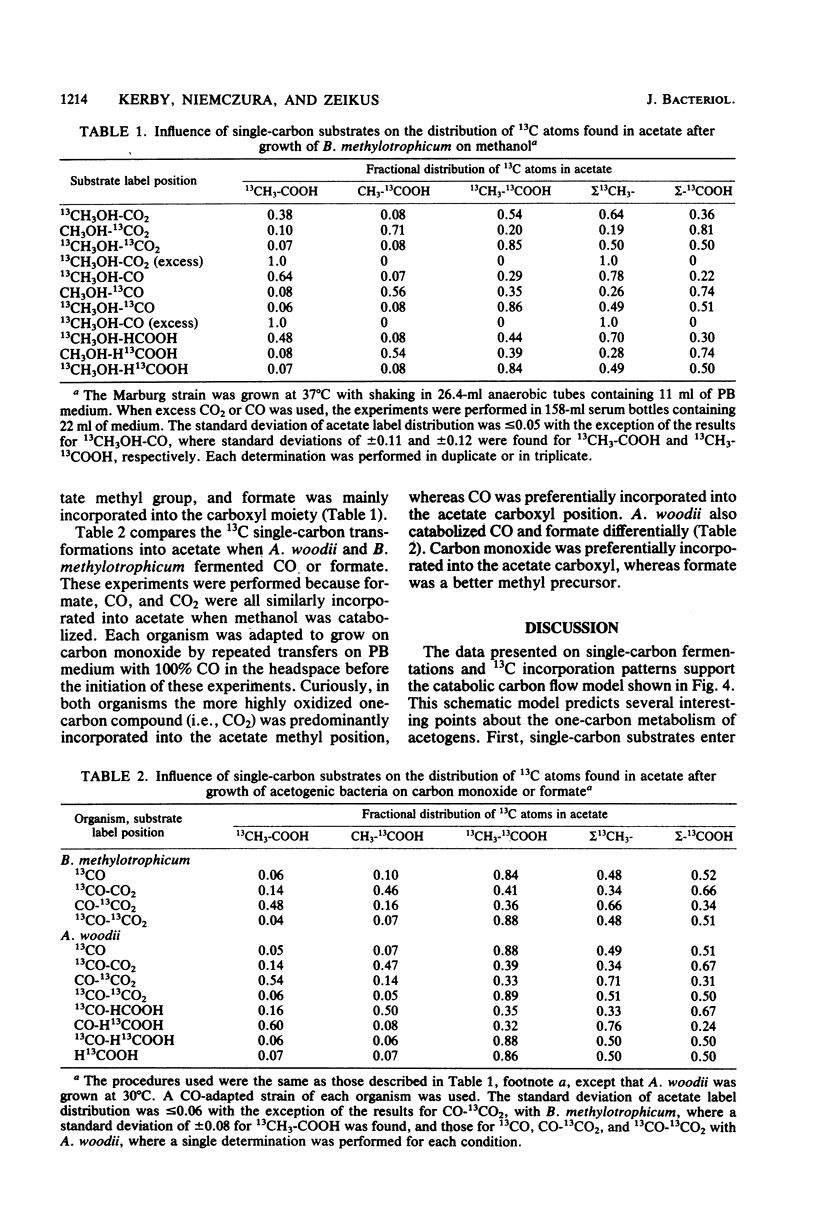
The catabolism of methanol, formate, or carbon monoxide to acetate or butyrate or both was examined in two acetogenic bacteria. Butyribacterium methylotrophicum simultaneously transformed methanol and formate mainly to butyrate with concomitant H2 and CO2 production and consumption. In contrast, methanol plus CO was primarily converted to acetate, and only slight amounts of CO2 were produced. In vivo 13C nuclear magnetic resonance analysis of [13C]methanol transformation by B. methylotrophicum indicated that methanol was predominantly incorporated into the methyl of acetate. 13CO2 was produced and then consumed, and butyrate was formed from the condensation of two acetate precursors. The analysis of the position of acetate labeled by a given 13C single-carbon substrate when B. methylotrophicum or Acetobacterium woodii was grown in the presence of a second one-carbon substrate indicated two trends: when methanol was consumed, CO, CO2, or formate predominantly labeled the acetate carboxyl; when CO was consumed, CO2 and formate were principally funneled into the acetate methyl group, and CO remained a better carboxyl precursor. These data suggest a model of acetate synthesis via the combined operation of two readily reversible single-carbon pathways which are linked by CO2.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andreesen J. R., Ljungdahl L. G. Nicotinamide adenine dinucleotide phosphate-dependent formate dehydrogenase from Clostridium thermoaceticum: purification and properties. J Bacteriol. 1974 Oct;120(1):6–14. doi: 10.1128/jb.120.1.6-14.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreesen J. R., Schaupp A., Neurauter C., Brown A., Ljungdahl L. G. Fermentation of glucose, fructose, and xylose by Clostridium thermoaceticum: effect of metals on growth yield, enzymes, and the synthesis of acetate from CO 2 . J Bacteriol. 1973 May;114(2):743–751. doi: 10.1128/jb.114.2.743-751.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark J. E., Ragsdale S. W., Ljungdahl L. G., Wiegel J. Levels of enzymes involved in the synthesis of acetate from CO2 in Clostridium thermoautotrophicum. J Bacteriol. 1982 Jul;151(1):507–509. doi: 10.1128/jb.151.1.507-509.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Counotte G. H., Prins R. A., Janssen R. H., Debie M. J. Role of Megasphaera elsdenii in the Fermentation of dl-[2-C]lactate in the Rumen of Dairy Cattle. Appl Environ Microbiol. 1981 Oct;42(4):649–655. doi: 10.1128/aem.42.4.649-655.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curthoys N. P., Rabinowitz J. C. Formyltetrahydrofolate synthetase. Binding of folate substrates and kinetics of the reverse reaction. J Biol Chem. 1972 Apr 10;247(7):1965–1971. [PubMed] [Google Scholar]
- Decker K., Jungermann K., Thauer R. K. Energy production in anaerobic organisms. Angew Chem Int Ed Engl. 1970 Feb;9(2):138–158. doi: 10.1002/anie.197001381. [DOI] [PubMed] [Google Scholar]
- Drake H. L., Hu S. I., Wood H. G. Purification of carbon monoxide dehydrogenase, a nickel enzyme from Clostridium thermocaceticum. J Biol Chem. 1980 Aug 10;255(15):7174–7180. [PubMed] [Google Scholar]
- Drake H. L., Hu S. I., Wood H. G. Purification of five components from Clostridium thermoaceticum which catalyze synthesis of acetate from pyruvate and methyltetrahydrofolate. Properties of phosphotransacetylase. J Biol Chem. 1981 Nov 10;256(21):11137–11144. [PubMed] [Google Scholar]
- Drake H. L. Occurrence of nickel in carbon monoxide dehydrogenase from Clostridium pasteurianum and Clostridium thermoaceticum. J Bacteriol. 1982 Feb;149(2):561–566. doi: 10.1128/jb.149.2.561-566.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genthner B. R., Bryant M. P. Growth of Eubacterium limosum with Carbon Monoxide as the Energy Source. Appl Environ Microbiol. 1982 Jan;43(1):70–74. doi: 10.1128/aem.43.1.70-74.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genthner B. R., Davis C. L., Bryant M. P. Features of rumen and sewage sludge strains of Eubacterium limosum, a methanol- and H2-CO2-utilizing species. Appl Environ Microbiol. 1981 Jul;42(1):12–19. doi: 10.1128/aem.42.1.12-19.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu S. I., Drake H. L., Wood H. G. Synthesis of acetyl coenzyme A from carbon monoxide, methyltetrahydrofolate, and coenzyme A by enzymes from Clostridium thermoaceticum. J Bacteriol. 1982 Feb;149(2):440–448. doi: 10.1128/jb.149.2.440-448.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ljungdahl L., Irion E., Wood H. G. Total synthesis of acetate from CO2. I. Co-methylcobyric acid and CO-(methyl)-5-methoxybenzimidazolylcobamide as intermediates with Clostridium thermoaceticum. Biochemistry. 1965 Dec;4(12):2771–2780. doi: 10.1021/bi00888a030. [DOI] [PubMed] [Google Scholar]
- Lynd L. H., Zeikus J. G. Metabolism of H2-CO2, methanol, and glucose by Butyribacterium methylotrophicum. J Bacteriol. 1983 Mar;153(3):1415–1423. doi: 10.1128/jb.153.3.1415-1423.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynd L., Kerby R., Zeikus J. G. Carbon monoxide metabolism of the methylotrophic acidogen Butyribacterium methylotrophicum. J Bacteriol. 1982 Jan;149(1):255–263. doi: 10.1128/jb.149.1.255-263.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PINE L., BARKER H. A. Tracer experiments on the mechanism of acetate formation from carbon dioxide by Butyribacterium rettgeri. J Bacteriol. 1954 Aug;68(2):216–226. doi: 10.1128/jb.68.2.216-226.1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker D. J., Wu T. F., Wood H. G. Total synthesis of acetate from CO 2 : methyltetrahydrofolate, an intermediate, and a procedure for separation of the folates. J Bacteriol. 1971 Nov;108(2):770–776. doi: 10.1128/jb.108.2.770-776.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragsdale S. W., Clark J. E., Ljungdahl L. G., Lundie L. L., Drake H. L. Properties of purified carbon monoxide dehydrogenase from Clostridium thermoaceticum, a nickel, iron-sulfur protein. J Biol Chem. 1983 Feb 25;258(4):2364–2369. [PubMed] [Google Scholar]
- Schulman M., Ghambeer R. K., Ljungdahl L. G., Wood H. G. Total synthesis of acetate from CO2. VII. Evidence with Clostridium thermoaceticum that the carboxyl of acetate is derived from the carboxyl of pyruvate by transcarboxylation and not by fixation of CO2. J Biol Chem. 1973 Sep 25;248(18):6255–6261. [PubMed] [Google Scholar]
- Shulman R. G., Brown T. R., Ugurbil K., Ogawa S., Cohen S. M., den Hollander J. A. Cellular applications of 31P and 13C nuclear magnetic resonance. Science. 1979 Jul 13;205(4402):160–166. doi: 10.1126/science.36664. [DOI] [PubMed] [Google Scholar]
- Tanner R. S., Wolfe R. S., Ljungdahl L. G. Tetrahydrofolate enzyme levels in Acetobacterium woodii and their implication in the synthesis of acetate from CO2. J Bacteriol. 1978 May;134(2):668–670. doi: 10.1128/jb.134.2.668-670.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thauer R. K. CO(2)-reduction to formate by NADPH. The initial step in the total synthesis of acetate from CO(2) in Clostridium thermoaceticum. FEBS Lett. 1972 Oct 15;27(1):111–115. doi: 10.1016/0014-5793(72)80421-6. [DOI] [PubMed] [Google Scholar]
- Thauer R. K., Jungermann K., Decker K. Energy conservation in chemotrophic anaerobic bacteria. Bacteriol Rev. 1977 Mar;41(1):100–180. doi: 10.1128/br.41.1.100-180.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traore A. S., Hatchikian C. E., Belaich J. P., Le Gall J. Microcalorimetric studies of the growth of sulfate-reducing bacteria: energetics of Desulfovibrio vulgaris growth. J Bacteriol. 1981 Jan;145(1):191–199. doi: 10.1128/jb.145.1.191-199.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WOLIN E. A., WOLIN M. J., WOLFE R. S. FORMATION OF METHANE BY BACTERIAL EXTRACTS. J Biol Chem. 1963 Aug;238:2882–2886. [PubMed] [Google Scholar]
- WOOD H. G. A study of carbon dioxide fixation by mass determination of the types of C13-acetate. J Biol Chem. 1952 Feb;194(2):905–931. [PubMed] [Google Scholar]
- Waber L. J., Wood H. G. Mechanism of acetate synthesis from CO2 by Clostridium acidiurici. J Bacteriol. 1979 Nov;140(2):468–478. doi: 10.1128/jb.140.2.468-478.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welty F. K., Wood H. G. Purification of the "corrinoid" enzyme involved in the synthesis of acetate by Clostridium thermoaceticum. J Biol Chem. 1978 Aug 25;253(16):5832–5838. [PubMed] [Google Scholar]
- Zeikus J. G. Metabolism of one-carbon compounds by chemotrophic anaerobes. Adv Microb Physiol. 1983;24:215–299. doi: 10.1016/s0065-2911(08)60387-2. [DOI] [PubMed] [Google Scholar]


