Abstract
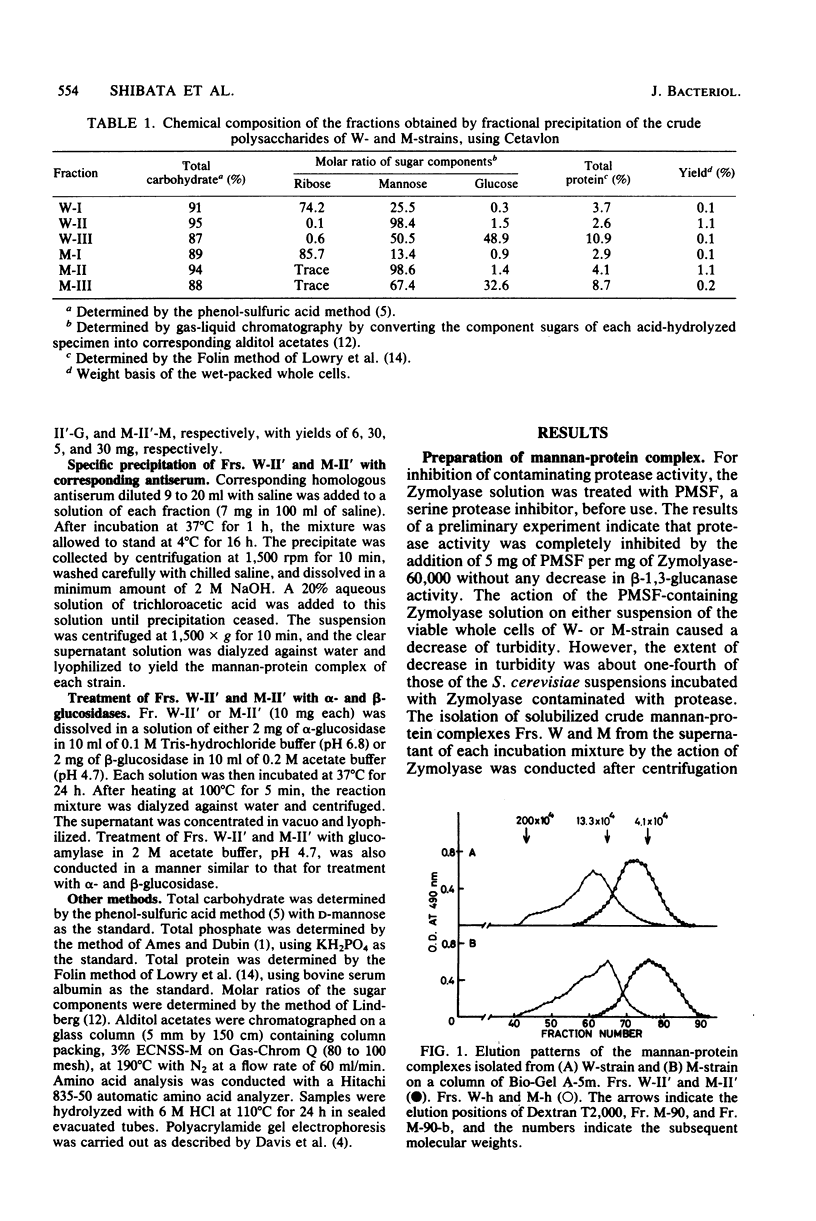
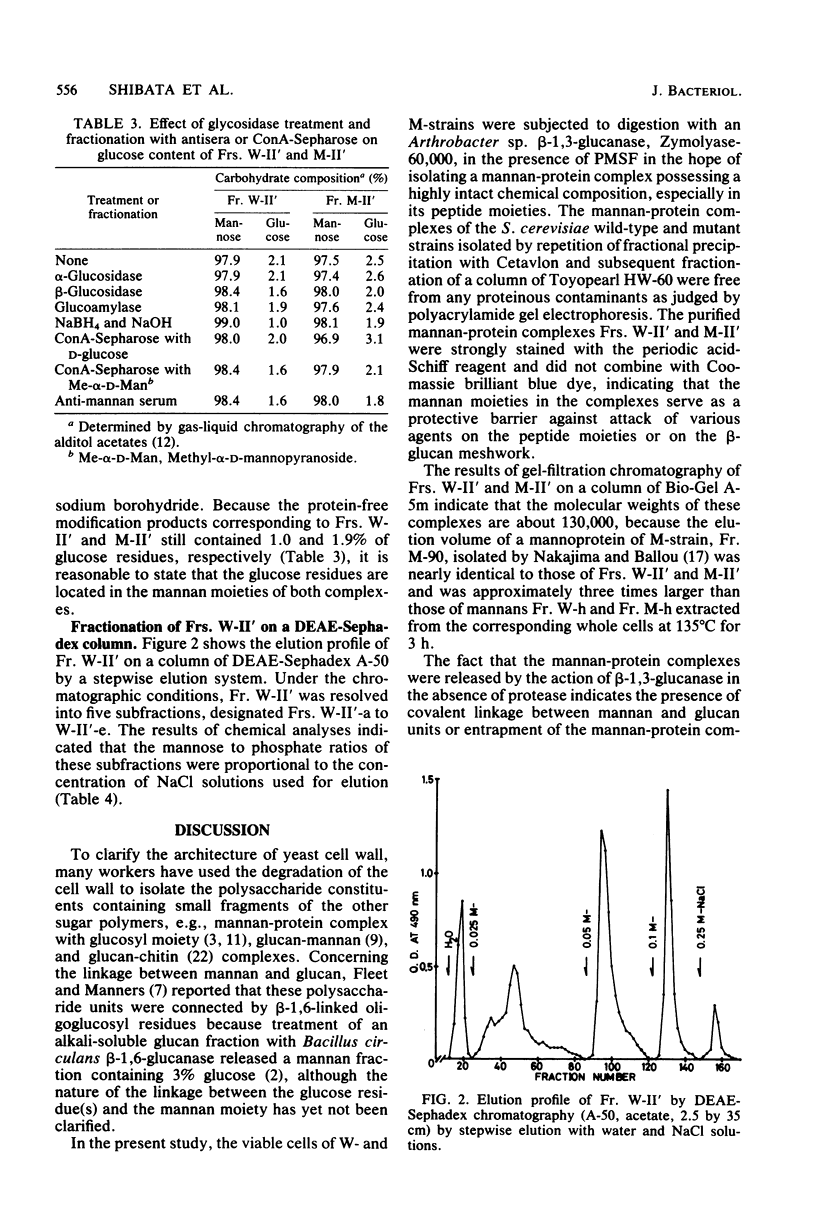
The viable whole cells of Saccharomyces cerevisiae X2180-1A wild type and its mannan mutant strain S. cerevisiae X2180-1A-5, were treated with an Arthrobacter sp. beta-1,3-glucanase in the presence of a serine protease inhibitor, phenyl-methylsulfonyl fluoride. Fractionation of the solubilized materials of each strain with Cetavlon (cetyltrimethylammonium bromide) yielded one mannan-protein complex. Molecular weights of these complexes were almost the same as that of the mannoprotein of the mutant strain prepared by Nakajima and Ballou, which had a molecular weight of 133,000 and were approximately three times larger than those of the mannans isolated from the same cells by hot-water extraction. Each mannan-protein complex contained up to 2% glucose residue, which was not removed by specific precipitation with anti-mannan sera or by affinity chromatography on a column of concanavalin A-Sepharose. Treatment of these complexes with alkaline NaBH4 produced peptide-free mannan containing small amounts of glucose nearly identical to those of the parent complexes. The above findings provide evidence that the glucose residues exist in a covalently linked form to the mannan moiety. Fractionation of the mannan-protein complex of the S. cerevisiae wild-type strain by DEAE-Sephadex chromatography yielded five subfractions of different phosphate content, indicating that these highly intact mannan-protein complexes were of heterogeneous material consisting of many molecular species of different phosphate content.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AMES B. N., DUBIN D. T. The role of polyamines in the neutralization of bacteriophage deoxyribonucleic acid. J Biol Chem. 1960 Mar;235:769–775. [PubMed] [Google Scholar]
- Bacon J. S., Gordon A. H., Jones D., Taylor I. F., Webley D. M. The separation of beta-glucanases produced by Cytophaga johnsonii and their role in the lysis of yeast cell walls. Biochem J. 1970 Nov;120(1):67–78. doi: 10.1042/bj1200067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer H., Horisberger M., Bush D. A., Sigarlakie E. Mannan as a major component of the bud scars of Saccharomyces cerevisiae. Arch Mikrobiol. 1972;85(3):202–208. doi: 10.1007/BF00408845. [DOI] [PubMed] [Google Scholar]
- Davis C. H., Schliselfeld L. H., Wolf D. P., Leavitt C. A., Krebs E. G. Interrelationships among glycogen phosphorylase isozymes. J Biol Chem. 1967 Oct 25;242(20):4824–4833. [PubMed] [Google Scholar]
- EDDY A. A. [The structure of the yeast cell wall. II. Degradative studies with enzymes]. Proc R Soc Lond B Biol Sci. 1958 Dec 17;149(936):425–440. doi: 10.1098/rspb.1958.0085. [DOI] [PubMed] [Google Scholar]
- Fleet G. H., Manners D. J. The enzymic degradation of an alkali-soluble glucan from the cell walls of Saccharomyces cerevisiae. J Gen Microbiol. 1977 Feb;98(2):315–327. doi: 10.1099/00221287-98-2-315. [DOI] [PubMed] [Google Scholar]
- KESSLER G., NICKERSON W. J. Glucomannan-protein complexes from cell walls of yeasts. J Biol Chem. 1959 Sep;234:2281–2285. [PubMed] [Google Scholar]
- KORN E. D., NORTHCOTE D. H. Physical and chemical properties of polysaccharides and glycoproteins of the yeast-cell wall. Biochem J. 1960 Apr;75:12–17. doi: 10.1042/bj0750012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kidby D. K., Davies R. Invertase and disulphide bridges in the yeast wall. J Gen Microbiol. 1970 Jun;61(3):327–333. doi: 10.1099/00221287-61-3-327. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lloyd K. O. Isolation, characterization, and partial structure of peptido galactomannans from the yeast form of Cladosporium werneckii. Biochemistry. 1970 Aug 18;9(17):3446–3453. doi: 10.1021/bi00819a025. [DOI] [PubMed] [Google Scholar]
- McLellan W. L., Jr, Lampen J. O. Phosphomannanase (PR-factor), an enzyme required for the formation of yeast protoplasts. J Bacteriol. 1968 Mar;95(3):967–974. doi: 10.1128/jb.95.3.967-974.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakajima T., Ballou C. E. Characterization of the carbohydrate fragments obtained from Saccharomyces cerevisiae mannan by alkaline degradation. J Biol Chem. 1974 Dec 10;249(23):7679–7684. [PubMed] [Google Scholar]
- Nakajima T., Ballou C. E. Structure of the linkage region between the polysaccharide and protein parts of Saccharomyces cerevisiae mannan. J Biol Chem. 1974 Dec 10;249(23):7685–7694. [PubMed] [Google Scholar]
- Okubo Y., Ichikawa T., Suzuki S. Relationship between phosphate content and immunochemical properties of subfractions of bakers' yeast mannan. J Bacteriol. 1978 Oct;136(1):63–68. doi: 10.1128/jb.136.1.63-68.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okubo Y., Shibata N., Ichikawa T., Chaki S., Suzuki S. Immunochemical study on bakers' yeast mannan prepared by fractional precipitation with cetyltrimethylammonium bromide. Arch Biochem Biophys. 1981 Nov;212(1):204–215. doi: 10.1016/0003-9861(81)90360-x. [DOI] [PubMed] [Google Scholar]
- SMOGYI M. Notes on sugar determination. J Biol Chem. 1952 Mar;195(1):19–23. [PubMed] [Google Scholar]
- Sentandreu R., Northcote D. H. The structure of a glycopeptide isolated from the yeast cell wall. Biochem J. 1968 Sep;109(3):419–432. doi: 10.1042/bj1090419. [DOI] [PMC free article] [PubMed] [Google Scholar]


