Abstract
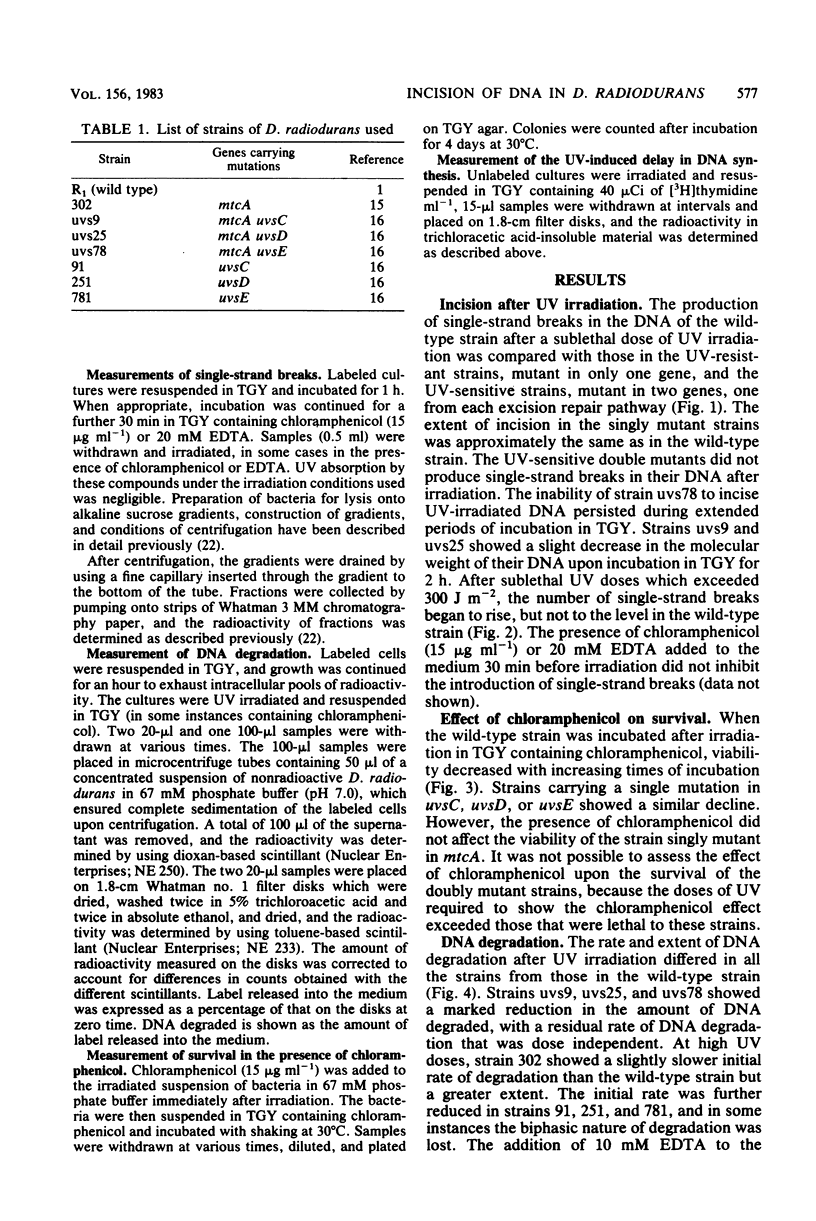
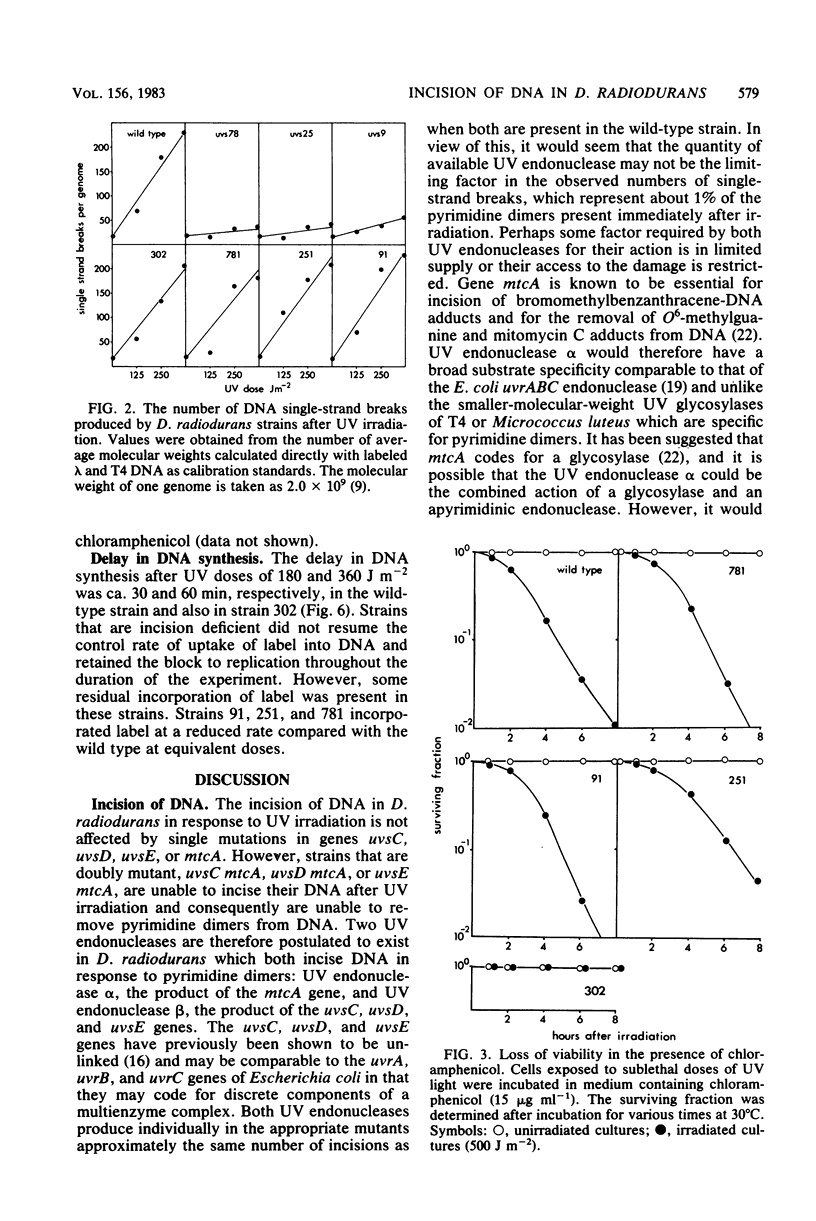
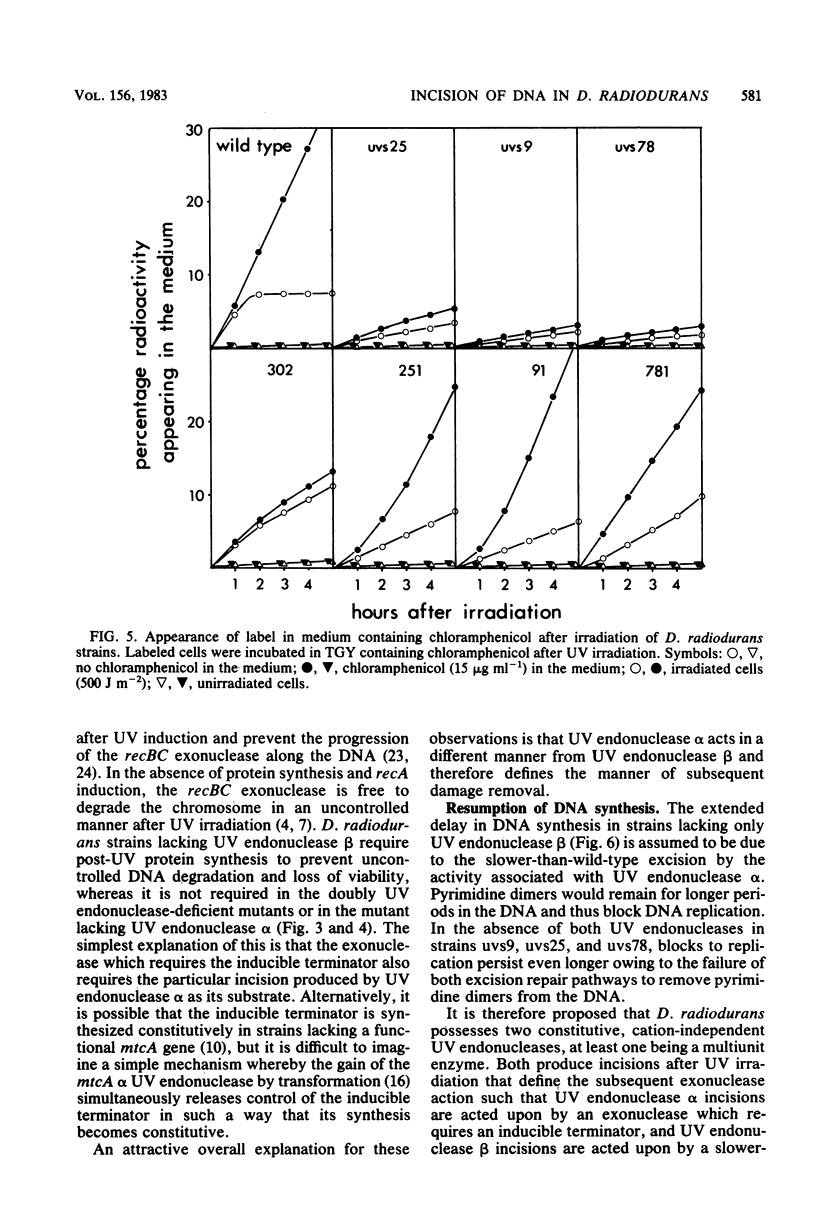
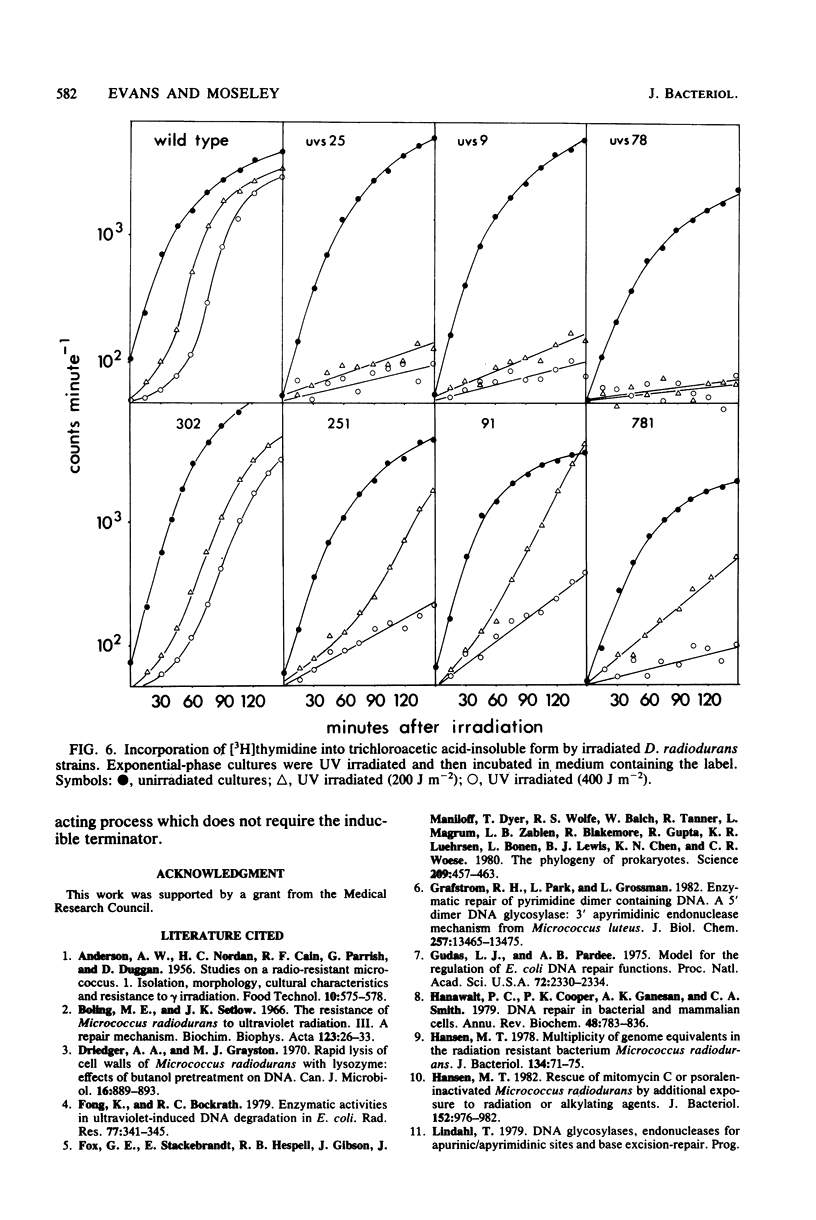
In Deinococcus radiodurans, the genes uvsC, uvsD, uvsE, and mtcA are all involved in the single-strand incision of UV-irradiated DNA, and mutations in at least two of them were required to produce an incisionless strain. One mutation must be in mtcA and one in uvsC, uvsD, or uvsE. Strains carrying single mutations in any one of the genes can incise DNA to the same extent as the wild-type strain. Neither the presence of EDTA nor the absence of protein synthesis affected the incision step. Strains deficient in DNA incision have greatly reduced DNA degradation after UV irradiation, and upon addition of chloramphenicol to the postirradiation medium, they do not undergo excessive DNA degradation as is seen in the wild-type strain and strains singly mutant in uvsC, uvsD, or uvsE. The strain singly mutant in mtcA also lacked chloramphenicol-enhanced DNA degradation and loss of viability but behaved similarly to the wild-type strain with respect to resumption of DNA synthesis and DNA degradation in the absence of chloramphenicol. It is proposed that two constitutive, cation-independent UV endonucleases are present in D. radiodurans: UV endonuclease alpha (the product of the mtcA gene), which incises in response to pyrimidine dimers, mitomycin C cross-links, bromomethylbenzanthracene adducts, and other alkylation damage, and UV endonuclease beta (the product of the uvsC, uvsD, and uvsE genes), which incises only in response to pyrimidine dimers. Both endonucleases have associated exonuclease activity.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boling M. E., Setlow J. K. The resistance of Micrococcus radiodurans to ultraviolet radiation. 3. A repair mechanism. Biochim Biophys Acta. 1966 Jul 20;123(1):26–33. doi: 10.1016/0005-2787(66)90155-9. [DOI] [PubMed] [Google Scholar]
- Driedger A. A., Grayston M. J. Rapid lysis of cell walls of Micrococcus radiodurans with lysozyme: effects of butanol pretreatment on DNA. Can J Microbiol. 1970 Sep;16(9):889–893. doi: 10.1139/m70-151. [DOI] [PubMed] [Google Scholar]
- Fong K., Bockrath R. C. Enzymatic activities in ultraviolet-induced DNA degradation in Escherichia coli. Radiat Res. 1979 Feb;77(2):341–345. [PubMed] [Google Scholar]
- Fox G. E., Stackebrandt E., Hespell R. B., Gibson J., Maniloff J., Dyer T. A., Wolfe R. S., Balch W. E., Tanner R. S., Magrum L. J. The phylogeny of prokaryotes. Science. 1980 Jul 25;209(4455):457–463. doi: 10.1126/science.6771870. [DOI] [PubMed] [Google Scholar]
- Grafstrom R. H., Park L., Grossman L. Enzymatic repair of pyrimidine dimer-containing DNA. A 5' dimer DNA glycosylase: 3'-apyrimidinic endonuclease mechanism from Micrococcus luteus. J Biol Chem. 1982 Nov 25;257(22):13465–13474. [PubMed] [Google Scholar]
- Gudas L. J., Pardee A. B. Model for regulation of Escherichia coli DNA repair functions. Proc Natl Acad Sci U S A. 1975 Jun;72(6):2330–2334. doi: 10.1073/pnas.72.6.2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanawalt P. C., Cooper P. K., Ganesan A. K., Smith C. A. DNA repair in bacteria and mammalian cells. Annu Rev Biochem. 1979;48:783–836. doi: 10.1146/annurev.bi.48.070179.004031. [DOI] [PubMed] [Google Scholar]
- Hansen M. T. Multiplicity of genome equivalents in the radiation-resistant bacterium Micrococcus radiodurans. J Bacteriol. 1978 Apr;134(1):71–75. doi: 10.1128/jb.134.1.71-75.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen M. T. Rescue of mitomycin C- or psoralen-inactivated Micrococcus radiodurans by additional exposure to radiation or alkylating agents. J Bacteriol. 1982 Dec;152(3):976–982. doi: 10.1128/jb.152.3.976-982.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindahl T. DNA glycosylases, endonucleases for apurinic/apyrimidinic sites, and base excision-repair. Prog Nucleic Acid Res Mol Biol. 1979;22:135–192. doi: 10.1016/s0079-6603(08)60800-4. [DOI] [PubMed] [Google Scholar]
- Mackay V., Linn S. Selective inhibition of the dnase activity of the recBC enzyme by the DNA binding protein from Escherichia coli. J Biol Chem. 1976 Jun 25;251(12):3716–3719. [PubMed] [Google Scholar]
- McMillan S., Edenberg H. J., Radany E. H., Friedberg R. C., Friedberg E. C. den V gene of bacteriophage T4 codes for both pyrimidine dimer-DNA glycosylase and apyrimidinic endonuclease activities. J Virol. 1981 Oct;40(1):211–223. doi: 10.1128/jvi.40.1.211-223.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moseley B. E., Copland H. F. Four mutants of Micrococcus radiodurans defective in the ability to repair DNA damaged by mitomycin-C, two of which have wild-type resistance to ultraviolet radiation. Mol Gen Genet. 1978 Apr 17;160(3):331–337. doi: 10.1007/BF00332977. [DOI] [PubMed] [Google Scholar]
- Moseley B. E., Copland H. J. Isolation and properties of a recombination-deficient mutant of Micrococcus radiodurans. J Bacteriol. 1975 Feb;121(2):422–428. doi: 10.1128/jb.121.2.422-428.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moseley B. E., Evans D. M. Isolation and properties of strains of Micrococcus (Deinococcus) radiodurans unable to excise ultraviolet light-induced pyrimidine dimers from DNA: evidence for two excision pathways. J Gen Microbiol. 1983 Aug;129(8):2437–2445. doi: 10.1099/00221287-129-8-2437. [DOI] [PubMed] [Google Scholar]
- Moseley B. E., Mattingly A., Copland H. J. Sensitization to radiation by loss of recombination ability in a temperature-sensitive DNA mutant of Micrococcus radiodurans held at its restrictive temperature. J Gen Microbiol. 1972 Sep;72(2):329–338. doi: 10.1099/00221287-72-2-329. [DOI] [PubMed] [Google Scholar]
- Pollard E. C., Randall E. P. Studies on the inducible inhibitor of radiation-induced DNA degradation of Escherichia coli. Radiat Res. 1973 Aug;55(2):265–279. [PubMed] [Google Scholar]
- Rupp W. D., Sancar A., Sancar G. B. Properties and regulation of the UVRABC endonuclease. Biochimie. 1982 Aug-Sep;64(8-9):595–598. doi: 10.1016/s0300-9084(82)80094-1. [DOI] [PubMed] [Google Scholar]
- Sancar A., Rupp W. D. A novel repair enzyme: UVRABC excision nuclease of Escherichia coli cuts a DNA strand on both sides of the damaged region. Cell. 1983 May;33(1):249–260. doi: 10.1016/0092-8674(83)90354-9. [DOI] [PubMed] [Google Scholar]
- Sweet D. M., Moseley B. E. The resistance of Micrococcus radiodurans to killing and mutation by agents which damage DNA. Mutat Res. 1976 Feb;34(2):175–186. doi: 10.1016/0027-5107(76)90122-6. [DOI] [PubMed] [Google Scholar]
- Tempest P. R., Moseley B. E. Defective excision repair in a mutant of Micrococcus radiodurans hypermutable by some monofunctional alkylating agents. Mol Gen Genet. 1980;179(1):191–199. doi: 10.1007/BF00268463. [DOI] [PubMed] [Google Scholar]
- Willetts N. S., Clark A. J. Characteristics of some multiply recombination-deficient strains of Escherichia coli. J Bacteriol. 1969 Oct;100(1):231–239. doi: 10.1128/jb.100.1.231-239.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams J. G., Shibata T., Radding C. M. Escherichia coli recA protein protects single-stranded DNA or gapped duplex DNA from degradation by RecBC DNase. J Biol Chem. 1981 Jul 25;256(14):7573–7582. [PubMed] [Google Scholar]


