Abstract
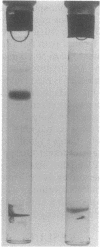
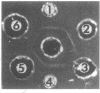
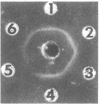
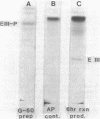
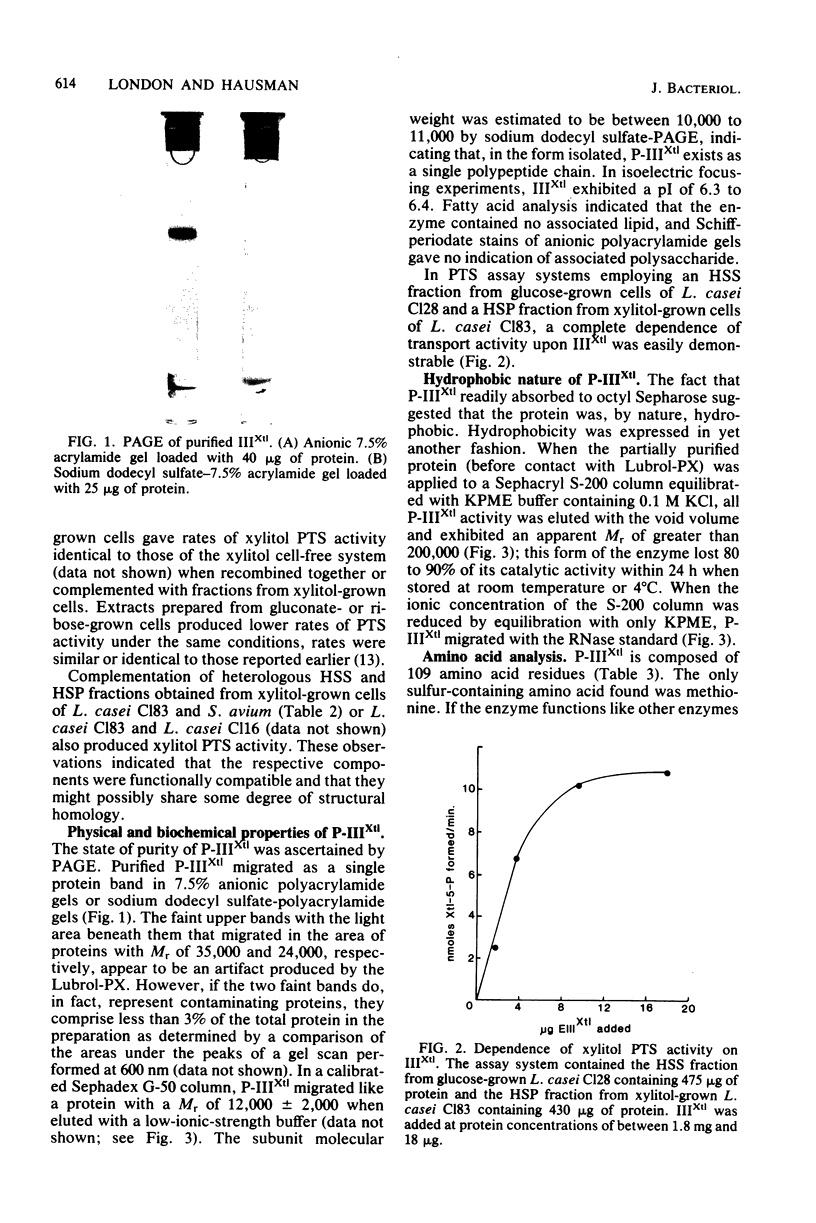
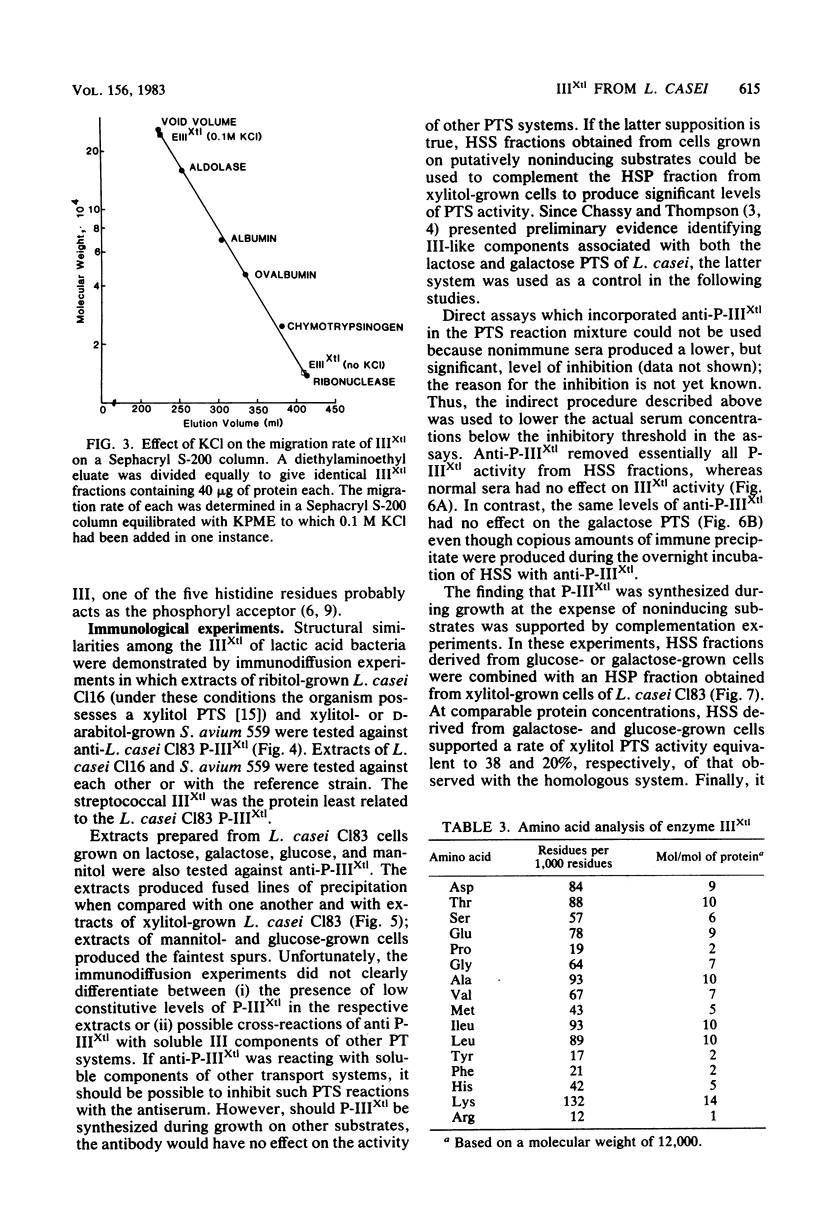
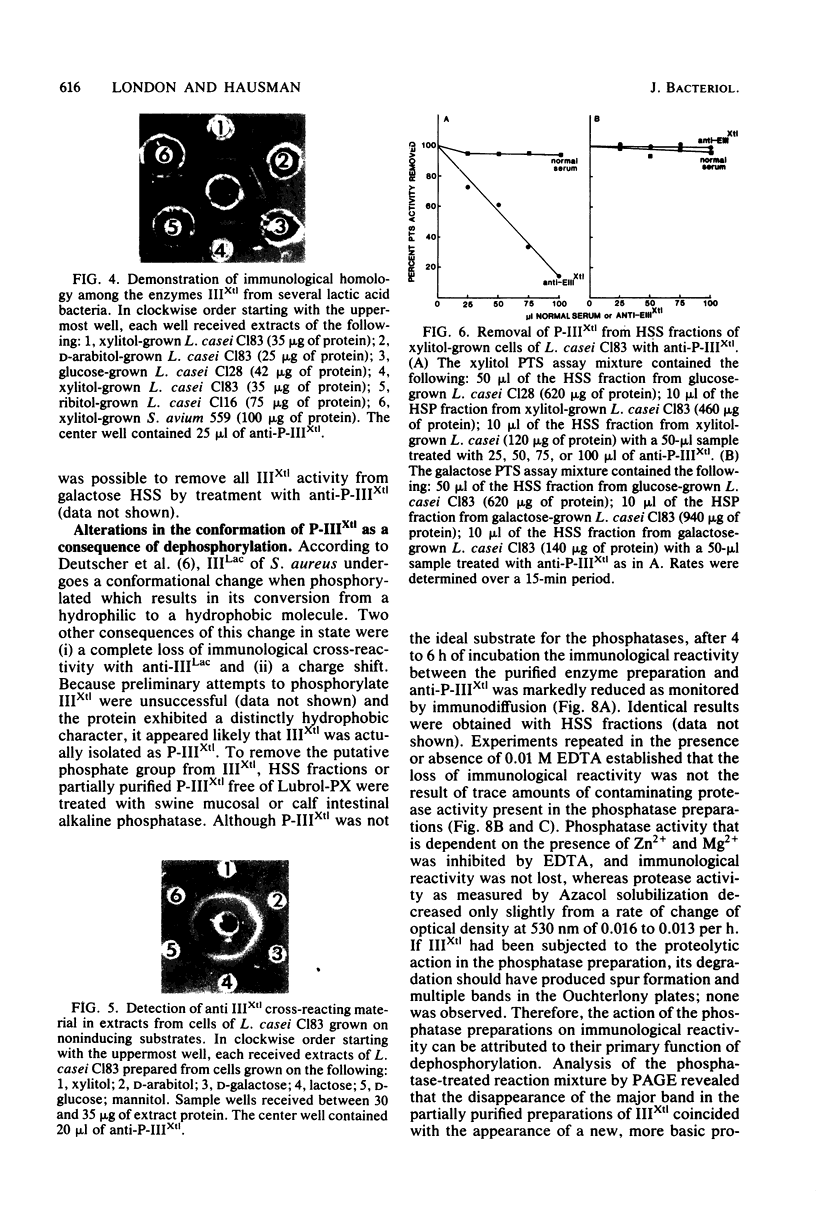
The phosphoenolpyruvate-dependent xylitol:phosphotransferase system of Lactobacillus casei strain C183 requires a small, soluble, substrate-specific protein for catalytic activity. Designated enzyme IIIXtl (or IIIXtl), the protein was purified to electrophoretic homogeneity and characterized. IIIXtl, as purified, is a single polypeptide composed of 109 amino acid residues. It has an estimated molecular weight of 12,000 and is hydrophobic in nature. The hydrophobicity of IIIXtl is apparently due to the fact that the enzyme was isolated as the phosphorylated phosphocarrier protein. Removal of the phosphate group with alkaline phosphatase results in the loss of immunological cross-reactivity with anti-P-IIIXtl and an alteration in charge. The L. casei C183 IIIXtl is antigenically related to enzymes IIIXtl in Streptococcus avium and other, genetically distinct strains of L. casei.
Full text
PDF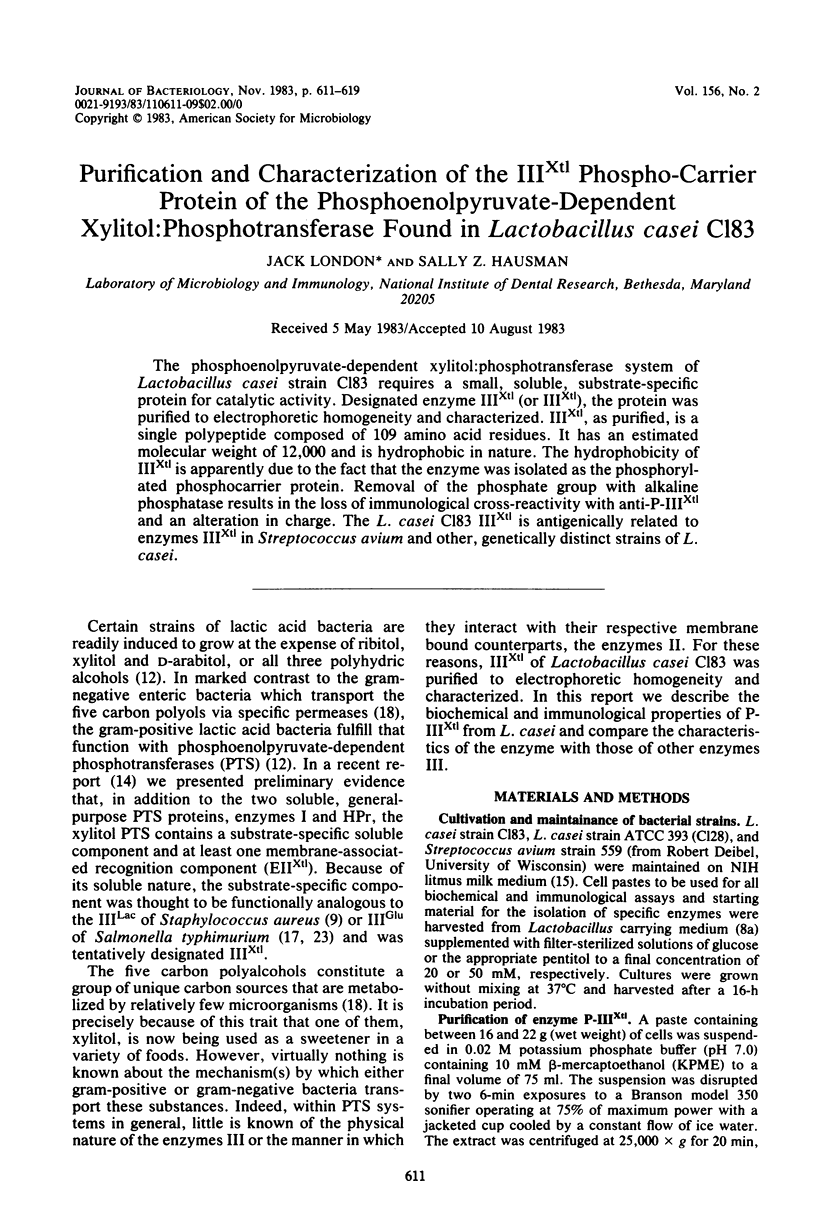




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrews P. Estimation of the molecular weights of proteins by Sephadex gel-filtration. Biochem J. 1964 May;91(2):222–233. doi: 10.1042/bj0910222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernsmann P., Alpert C. A., Muss P., Deutscher J., Hengstenberg W. The bacterial PEP-dependent phosphotransferase system mechanism of gluconate phosphorylation in Streptococcus faecalis. FEBS Lett. 1982 Feb 8;138(1):101–103. doi: 10.1016/0014-5793(82)80404-3. [DOI] [PubMed] [Google Scholar]
- Chassy B. M., Thompson J. Regulation and characterization of the galactose-phosphoenolpyruvate-dependent phosphotransferase system in Lactobacillus casei. J Bacteriol. 1983 Jun;154(3):1204–1214. doi: 10.1128/jb.154.3.1204-1214.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chassy B. M., Thompson J. Regulation of lactose-phosphoenolpyruvate-dependent phosphotransferase system and beta-D-phosphogalactoside galactohydrolase activities in Lactobacillus casei. J Bacteriol. 1983 Jun;154(3):1195–1203. doi: 10.1128/jb.154.3.1195-1203.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- Deutscher J., Beyreuther K., Sobek H. M., Stüber K., Hengstenberg W. Phosphoenolpyruvate-dependent phosphotransferase system of Staphylococcus aureus: factor IIIlac, a trimeric phospho-carrier protein that also acts as a phase transfer catalyst. Biochemistry. 1982 Sep 28;21(20):4867–4873. doi: 10.1021/bi00263a006. [DOI] [PubMed] [Google Scholar]
- Fairbanks G., Steck T. L., Wallach D. F. Electrophoretic analysis of the major polypeptides of the human erythrocyte membrane. Biochemistry. 1971 Jun 22;10(13):2606–2617. doi: 10.1021/bi00789a030. [DOI] [PubMed] [Google Scholar]
- Hays J. B., Simoni R. D., Roseman S. Sugar transport. V. A trimeric lactose-specific phosphocarrier protein of the Staphylococcus aureus phosphotransferase system. J Biol Chem. 1973 Feb 10;248(3):941–956. [PubMed] [Google Scholar]
- Kenyon C. N., Stanier R. Y. Possible evolutionary significance of polyunsaturated fatty acids in blue-green algae. Nature. 1970 Sep 12;227(5263):1164–1166. doi: 10.1038/2271164a0. [DOI] [PubMed] [Google Scholar]
- King J., Laemmli U. K. Polypeptides of the tail fibres of bacteriophage T4. J Mol Biol. 1971 Dec 28;62(3):465–477. doi: 10.1016/0022-2836(71)90148-3. [DOI] [PubMed] [Google Scholar]
- London J., Chace N. M. New pathway for the metabolism of pentitols. Proc Natl Acad Sci U S A. 1977 Oct;74(10):4296–4300. doi: 10.1073/pnas.74.10.4296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- London J., Chace N. M. Pentitol metabolism in Lactobacillus casei. J Bacteriol. 1979 Dec;140(3):949–954. doi: 10.1128/jb.140.3.949-954.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- London J., Hausman S. Xylitol-mediated transient inhibition of ribitol utilization by Lactobacillus casei. J Bacteriol. 1982 May;150(2):657–661. doi: 10.1128/jb.150.2.657-661.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- London J., Kline K. Aldolase of lactic acid bacteria: a case history in the use of an enzyme as an evolutionary marker. Bacteriol Rev. 1973 Dec;37(4):453–478. doi: 10.1128/br.37.4.453-478.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meadow N. D., Roseman S. Sugar transport by the bacterial phosphotransferase system. Isolation and characterization of a glucose-specific phosphocarrier protein (IIIGlc) from Salmonella typhimurium. J Biol Chem. 1982 Dec 10;257(23):14526–14537. [PubMed] [Google Scholar]
- Mortlock R. P. Catabolism of unnatural carbohydrates by micro-organisms. Adv Microb Physiol. 1976;13:1–53. doi: 10.1016/s0065-2911(08)60037-5. [DOI] [PubMed] [Google Scholar]
- Nairn A. C., Detre J. A., Casnellie J. E., Greengard P. Serum antibodies that distinguish between the phospho- and dephospho-forms of a phosphoprotein. Nature. 1982 Oct 21;299(5885):734–736. doi: 10.1038/299734a0. [DOI] [PubMed] [Google Scholar]
- Nowlan S. S., Deibel R. H. Group Q streptococci. I. Ecology, serology, physiology, and relationship to established enterococci. J Bacteriol. 1967 Aug;94(2):291–296. doi: 10.1128/jb.94.2.291-296.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PERRIN D. Immunological studies with genetically altered beta-galactosidases. Ann N Y Acad Sci. 1963 May 8;103:1058–1066. doi: 10.1111/j.1749-6632.1963.tb53757.x. [DOI] [PubMed] [Google Scholar]
- PIEZ K. A., MORRIS L. A modified procedure for the automatic analysis of amino acids. Anal Biochem. 1960 Nov;1:187–201. doi: 10.1016/0003-2697(60)90045-2. [DOI] [PubMed] [Google Scholar]
- Scholte B. J., Schuitema A. R., Postma P. W. Isolation of IIIGlc of the phosphoenolpyruvate-dependent glucose phosphotransferase system of Salmonella typhimurium. J Bacteriol. 1981 Oct;148(1):257–264. doi: 10.1128/jb.148.1.257-264.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson J. In vivo regulation of glycolysis and characterization of sugar: phosphotransferase systems in Streptococcus lactis. J Bacteriol. 1978 Nov;136(2):465–476. doi: 10.1128/jb.136.2.465-476.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]