Abstract
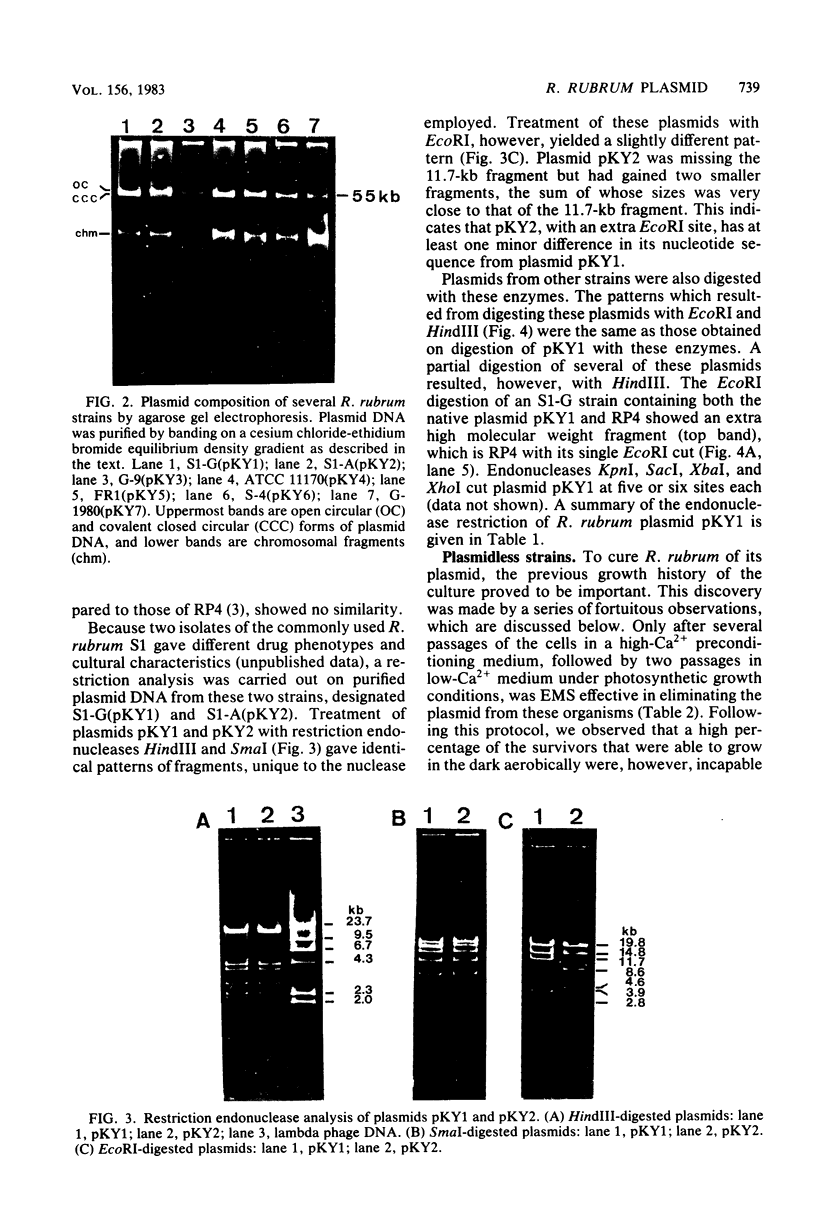
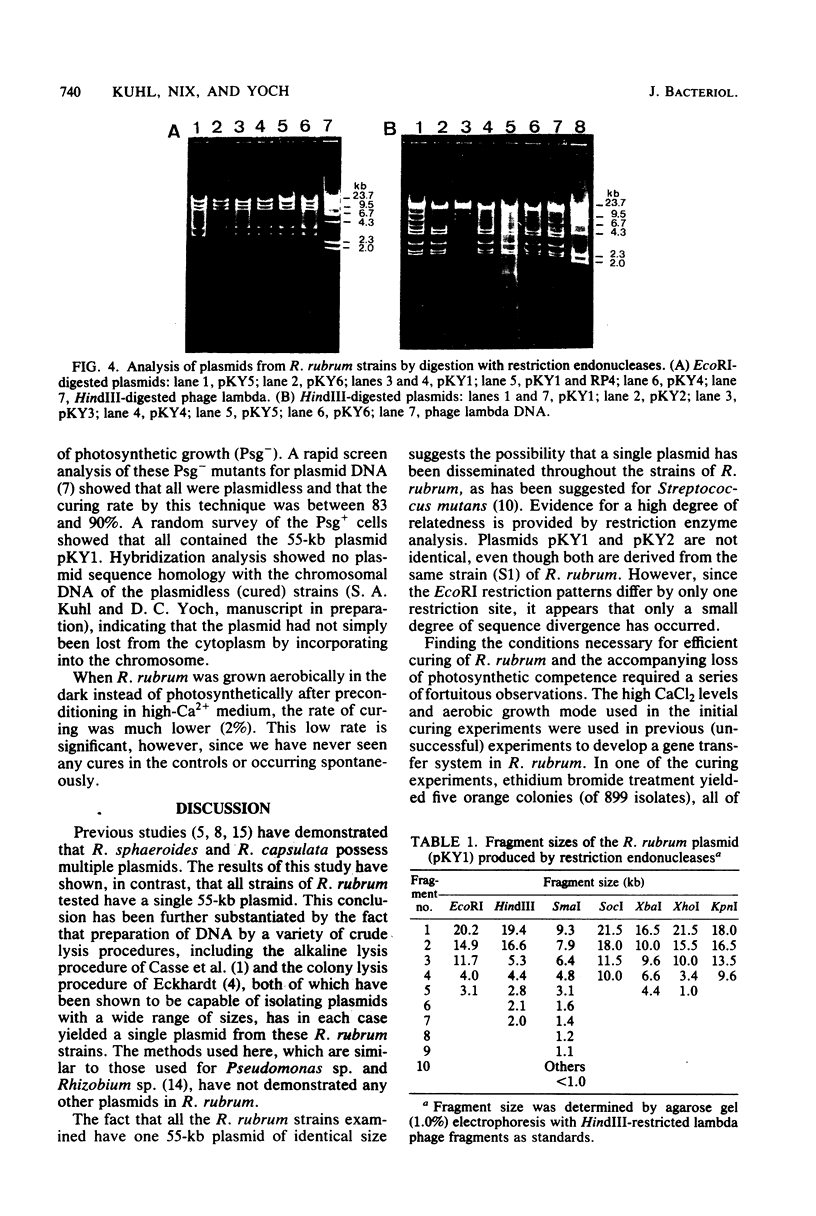
A single plasmid of 55 kilobases was found in crude cell lysates of each of nine strains of Rhodospirillum rubrum. Restriction endonuclease analysis showed identical fragment patterns with a given nuclease for all plasmids except one, for which an additional EcoRI site was observed. Elimination of the plasmid required that the cells be passaged several times in 25 mM calcium-containing medium, followed by at least two passages under photosynthetic growth conditions in low-calcium medium before treatment with ethyl methanesulfonate. The resulting plasmidless mutants only grew aerobically and were all incapable of pigment formation and photosynthetic growth, suggesting that plasmid DNA is required for photosynthetic competence in R. rubrum.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Eckhardt T. A rapid method for the identification of plasmid desoxyribonucleic acid in bacteria. Plasmid. 1978 Sep;1(4):584–588. doi: 10.1016/0147-619x(78)90016-1. [DOI] [PubMed] [Google Scholar]
- Gibson K. D., Niederman R. A. Characterization of two circular satellite species of deoxyribonucleic acid in Rhodopseudomonas spheroides. Arch Biochem Biophys. 1970 Dec;141(2):694–704. doi: 10.1016/0003-9861(70)90190-6. [DOI] [PubMed] [Google Scholar]
- Guerry P., LeBlanc D. J., Falkow S. General method for the isolation of plasmid deoxyribonucleic acid. J Bacteriol. 1973 Nov;116(2):1064–1066. doi: 10.1128/jb.116.2.1064-1066.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes D. S., Quigley M. A rapid boiling method for the preparation of bacterial plasmids. Anal Biochem. 1981 Jun;114(1):193–197. doi: 10.1016/0003-2697(81)90473-5. [DOI] [PubMed] [Google Scholar]
- Humphreys G. O., Willshaw G. A., Anderson E. S. A simple method for the preparation of large quantities of pure plasmid DNA. Biochim Biophys Acta. 1975 Apr 2;383(4):457–463. doi: 10.1016/0005-2787(75)90318-4. [DOI] [PubMed] [Google Scholar]
- Macrina F. L., Scott C. L. Evidence for a disseminated plasmid in Streptococcus mutans. Infect Immun. 1978 Apr;20(1):296–302. doi: 10.1128/iai.20.1.296-302.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marrs B. Mobilization of the genes for photosynthesis from Rhodopseudomonas capsulata by a promiscuous plasmid. J Bacteriol. 1981 Jun;146(3):1003–1012. doi: 10.1128/jb.146.3.1003-1012.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyers J. A., Sanchez D., Elwell L. P., Falkow S. Simple agarose gel electrophoretic method for the identification and characterization of plasmid deoxyribonucleic acid. J Bacteriol. 1976 Sep;127(3):1529–1537. doi: 10.1128/jb.127.3.1529-1537.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ORMEROD J. G., ORMEROD K. S., GEST H. Light-dependent utilization of organic compounds and photoproduction of molecular hydrogen by photosynthetic bacteria; relationships with nitrogen metabolism. Arch Biochem Biophys. 1961 Sep;94:449–463. doi: 10.1016/0003-9861(61)90073-x. [DOI] [PubMed] [Google Scholar]
- Rosenberg C., Casse-Delbart F., Dusha I., David M., Boucher C. Megaplasmids in the plant-associated bacteria Rhizobium meliloti and Pseudomonas solanacearum. J Bacteriol. 1982 Apr;150(1):402–406. doi: 10.1128/jb.150.1.402-406.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suyama Y., Gibson J. Satellite DNA in photosynthetic bacteria. Biochem Biophys Res Commun. 1966 Aug 23;24(4):549–553. doi: 10.1016/0006-291x(66)90355-x. [DOI] [PubMed] [Google Scholar]
- Thomas M., Davis R. W. Studies on the cleavage of bacteriophage lambda DNA with EcoRI Restriction endonuclease. J Mol Biol. 1975 Jan 25;91(3):315–328. doi: 10.1016/0022-2836(75)90383-6. [DOI] [PubMed] [Google Scholar]
- Uffen R. L., Sybesma C., Wolfe R. S. Mutants of Rhodospirrillum rubrum obtained after long-term anaerobic, dark growth. J Bacteriol. 1971 Dec;108(3):1348–1356. doi: 10.1128/jb.108.3.1348-1356.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]