Abstract
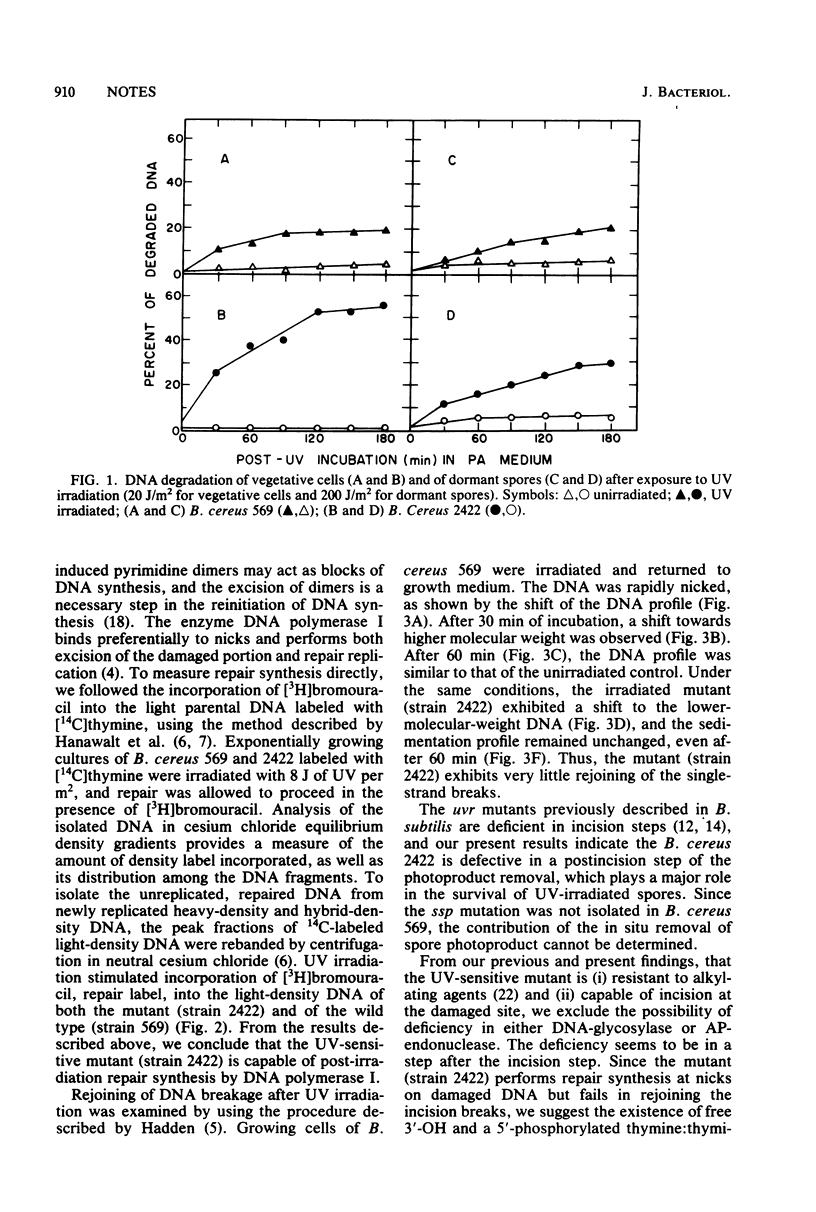
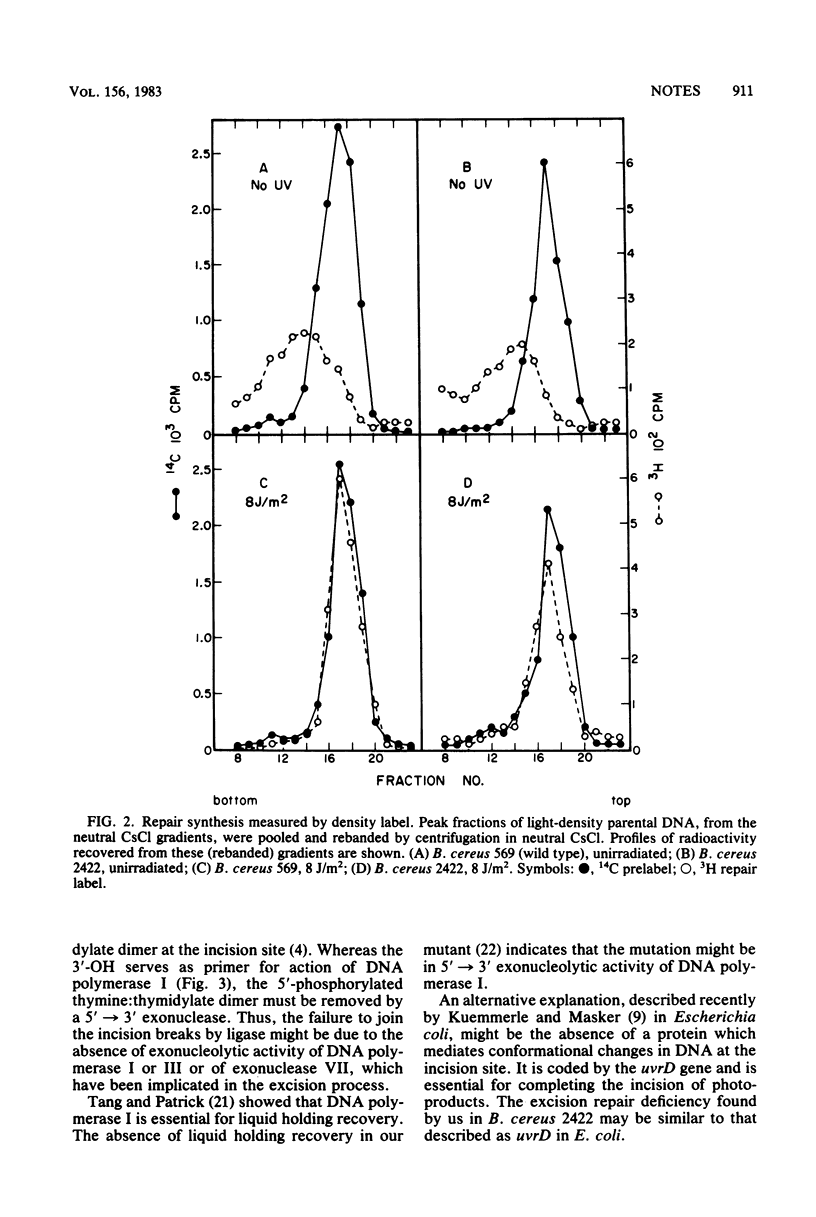
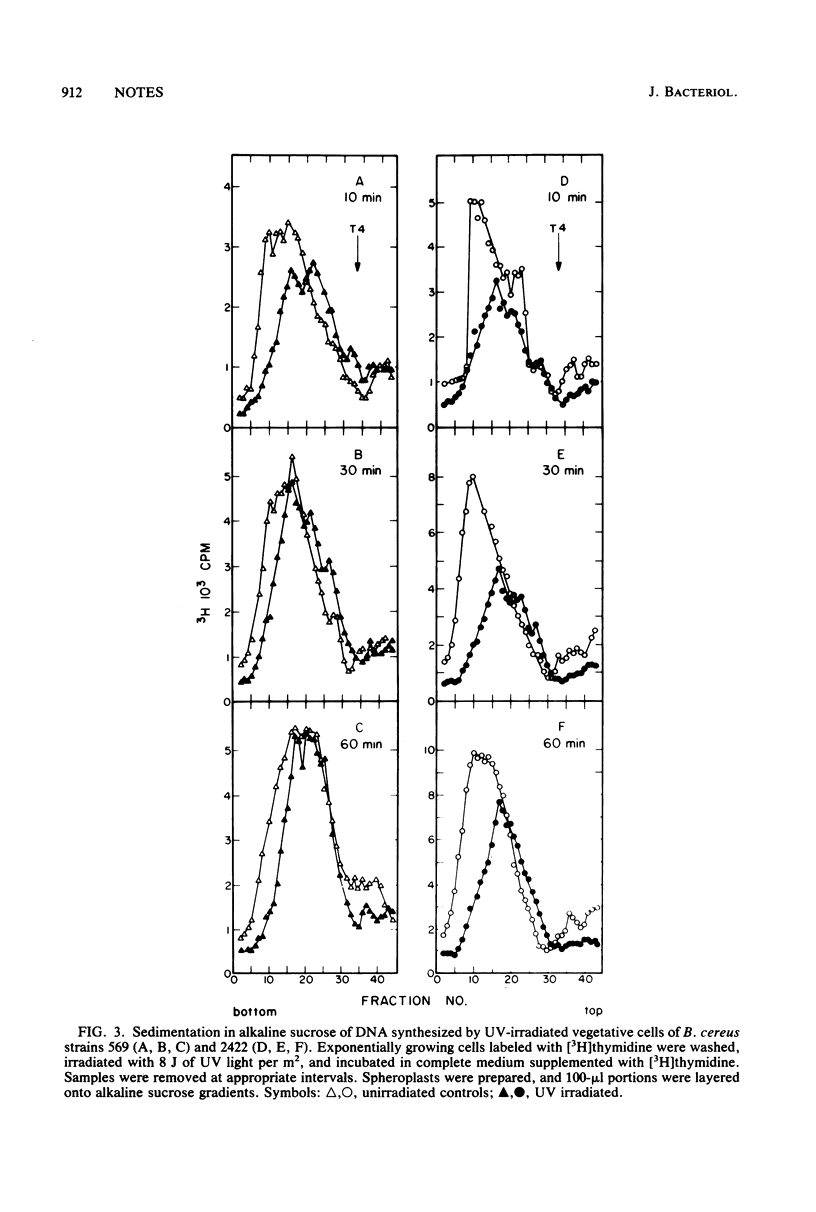
An excision-defective mutant of Bacillus cereus 569 is normal in incision and repair synthesis, but rejoining of incision breaks is defective, resulting in accumulation of low-molecular-weight DNA after UV irradiation. The defect in removal of photoproducts by exonuclease after incision renders both vegetative cells and dormant spores of the mutant sensitive to UV. A similarity is indicated to the uvrD mutation described recently in Escherichia coli.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brown N. C. Inhibition of bacterial DNA replication by 6-(p-hydroxyphenylazo)-uracil: differential effect on repair and semi-conservative synthesis in Bacillus subtilis. J Mol Biol. 1971 Jul 14;59(1):1–16. doi: 10.1016/0022-2836(71)90409-8. [DOI] [PubMed] [Google Scholar]
- EMMERSON P. T., HOWARD-FLANDERS P. POST-IRRADIATION DEGRADATION OF DNA FOLLOWING EXPOSURE OF UV-SENSITIVE AND RESISTANT BACTERIA TO X-RAYS. Biochem Biophys Res Commun. 1965 Jan 4;18:24–29. doi: 10.1016/0006-291x(65)90876-4. [DOI] [PubMed] [Google Scholar]
- Gossard F., Verly W. G. Properties of the main endonuclease specific for apurinic sites of Escherichia coli (endonuclease VI). Mechanism of apurinic site excision from DNA. Eur J Biochem. 1978 Jan 16;82(2):321–332. doi: 10.1111/j.1432-1033.1978.tb12026.x. [DOI] [PubMed] [Google Scholar]
- Grossman L. Enzymes involved in the repair of damaged DNA. Arch Biochem Biophys. 1981 Oct 15;211(2):511–522. doi: 10.1016/0003-9861(81)90485-9. [DOI] [PubMed] [Google Scholar]
- Grossman L., Riazuddin S., Haseltine W. A., Lindan C. Nucleotide excision repair of damaged DNA. Cold Spring Harb Symp Quant Biol. 1979;43(Pt 2):947–955. doi: 10.1101/sqb.1979.043.01.104. [DOI] [PubMed] [Google Scholar]
- Hadden C. T. Repair and subsequent fragmentation of deoxyribonucleic acid in ultraviolet-irradiated Bacillus subtilis recA. J Bacteriol. 1977 Dec;132(3):856–861. doi: 10.1128/jb.132.3.856-861.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanawalt P. C., Cooper P. K., Ganesan A. K., Smith C. A. DNA repair in bacteria and mammalian cells. Annu Rev Biochem. 1979;48:783–836. doi: 10.1146/annurev.bi.48.070179.004031. [DOI] [PubMed] [Google Scholar]
- Howard-Flanders P. DNA repair. Annu Rev Biochem. 1968;37:175–200. doi: 10.1146/annurev.bi.37.070168.001135. [DOI] [PubMed] [Google Scholar]
- Kuemmerle N. B., Masker W. E. Effect of the uvrD mutation on excision repair. J Bacteriol. 1980 May;142(2):535–546. doi: 10.1128/jb.142.2.535-546.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laval J., Laval F. Enzymology of DNA repair. IARC Sci Publ. 1980;(27):55–73. [PubMed] [Google Scholar]
- Munakata N. Genetic analysis of a mutant of Bacillus subtilis producingltraviolet-sensitive spores. Mol Gen Genet. 1969 Jul 3;104(3):258–263. doi: 10.1007/BF02539290. [DOI] [PubMed] [Google Scholar]
- Munakata N., Rupert C. S. Dark repair of DNA containing "spore photoproduct" in Bacillus subtilis. Mol Gen Genet. 1974 May 31;130(3):239–250. doi: 10.1007/BF00268802. [DOI] [PubMed] [Google Scholar]
- Munakata N., Rupert C. S. Effects of DNA-polymerase-defective and recombination-deficient mutations on the ultraviolet sensitivity of Bacillus subtilis spores. Mutat Res. 1975 Feb;27(2):157–169. doi: 10.1016/0027-5107(75)90075-5. [DOI] [PubMed] [Google Scholar]
- Munakata N., Rupert C. S. Genetically controlled removal of "spore photoproduct" from deoxyribonucleic acid of ultraviolet-irradiated Bacillus subtilis spores. J Bacteriol. 1972 Jul;111(1):192–198. doi: 10.1128/jb.111.1.192-198.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okubo S., Nakayama H. DNA synthesis after ultraviolet light irradiation in uv-sensitive mutants of Bacillus subtilis. Mutat Res. 1967 Sep-Oct;4(5):533–541. doi: 10.1016/0027-5107(67)90039-5. [DOI] [PubMed] [Google Scholar]
- Parodi S., Taningher M., Boero P., Santi L. Quantitative correlations amongst alkaline DNA fragmentation, DNA covalent binding, mutagenicity in the Ames test and carcinogenicity, for 21 compounds. Mutat Res. 1982 Mar;93(1):1–24. doi: 10.1016/0027-5107(82)90121-x. [DOI] [PubMed] [Google Scholar]
- Seeberg E., Rupp W. D., Strike P. Impaired incision of ultraviolet-irradiated deoxyribonucleic acid in uvrC mutants of Escherichia coli. J Bacteriol. 1980 Oct;144(1):97–104. doi: 10.1128/jb.144.1.97-104.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith K. C. Multiple pathways of DNA repair in bacteria and their roles in mutagenesis. Photochem Photobiol. 1978 Aug;28(2):121–129. doi: 10.1111/j.1751-1097.1978.tb07688.x. [DOI] [PubMed] [Google Scholar]
- Tang M. S., Patrick M. H. The role of DNA polymerase I in liquid holding recovery of UV-irradiated Escherichia coli. Photochem Photobiol. 1977 Sep;26(3):257–262. doi: 10.1111/j.1751-1097.1977.tb07482.x. [DOI] [PubMed] [Google Scholar]
- Youngs D. A., Smith K. C. Single-strand breaks in the DNA of the uvrA and uvrB strains of Escherichia coli K-12 after ultraviolet irradiation. Photochem Photobiol. 1976 Dec;24(6):533–541. doi: 10.1111/j.1751-1097.1976.tb06870.x. [DOI] [PubMed] [Google Scholar]


