Abstract
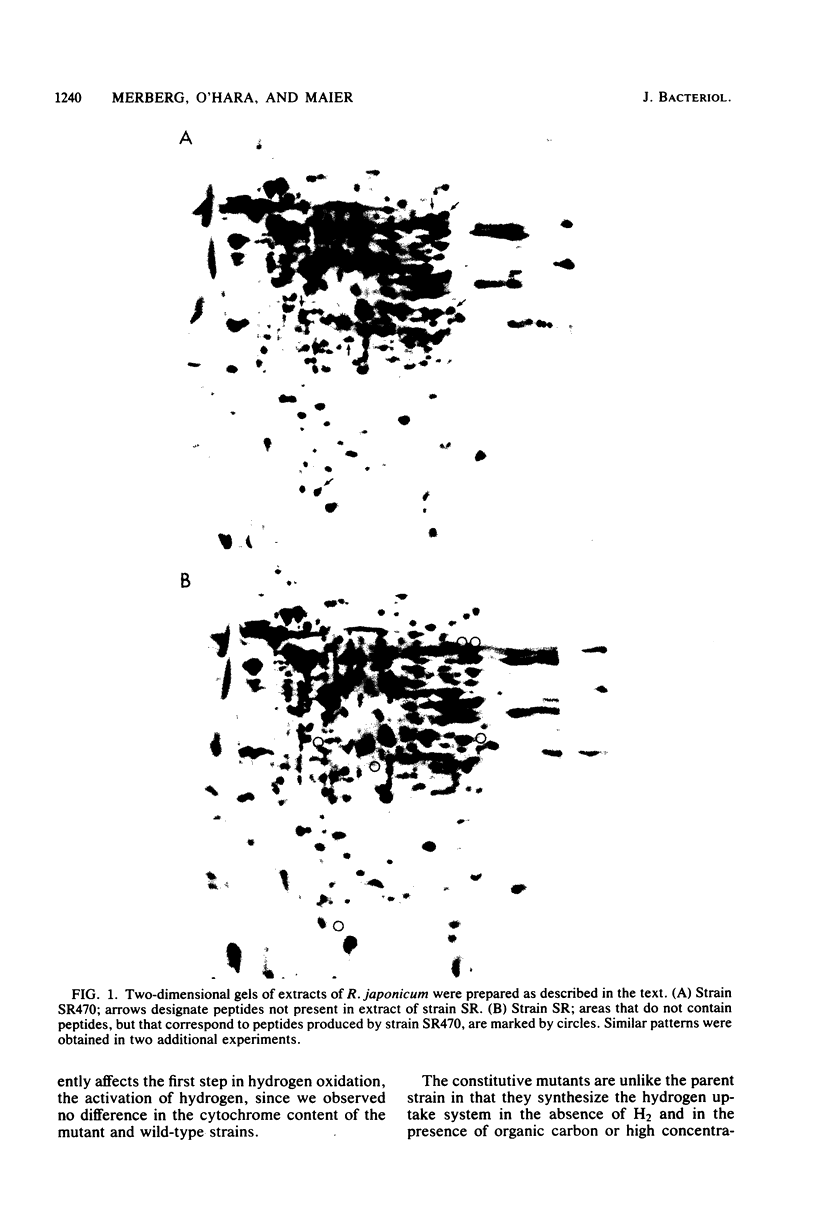
The synthesis of the H2 uptake system in free-living Rhizobium japonicum SR is repressed both by oxygen and by carbon substrates. Mutants selected for the ability to express hydrogenase in 10.0% partial pressure O2 were also less sensitive than the wild type to repression by carbon substrates such as arabinose, glycerol, gluconate, and succinate. The H2 uptake system in another class of mutants, previously shown to be hypersensitive to repression by O2, is also more sensitive to repression by carbon substrates. The oxygen- and carbon-insensitive mutants express the hydrogen uptake system during heterotrophic growth in the absence of hydrogen and thus can be considered constitutive (Hupc). The amount of cytochromes in the Hupc mutants is similar to that in the wild-type strain; however, the Hupc mutants contain greater methylene blue-dependent and O2-dependent hydrogenase activity, both as free-living cells and as bacteroids. Two-dimensional polyacrylamide gel electrophoresis revealed that during heterotrophic growth the Hupc mutant strain SR470 synthesized at least six peptides not found in the wild-type strain. The concentrations of cyclic AMP and guanosine tetraphosphate were similar in strain SR and the Hupc mutants during heterotrophic growth.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arp D. J., Burris R. H. Purification and properties of the particulate hydrogenase from the bacteroids of soybean root nodules. Biochim Biophys Acta. 1979 Oct 11;570(2):221–230. doi: 10.1016/0005-2744(79)90142-6. [DOI] [PubMed] [Google Scholar]
- Belitsky B., Kari C. Absence of accumulation of ppGpp and RNA during amino acid starvation in Rhizobium meliloti. J Biol Chem. 1982 May 10;257(9):4677–4679. [PubMed] [Google Scholar]
- Bishop P. E., Guevara J. G., Engelke J. A., Evans H. J. Relation between Glutamine Synthetase and Nitrogenase Activities in the Symbiotic Association between Rhizobium japonicum and Glycine max. Plant Physiol. 1976 Apr;57(4):542–546. doi: 10.1104/pp.57.4.542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botsford J. L. Cyclic nucleotides in procaryotes. Microbiol Rev. 1981 Dec;45(4):620–642. doi: 10.1128/mr.45.4.620-642.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowien B., Schlegel H. G. Physiology and biochemistry of aerobic hydrogen-oxidizing bacteria. Annu Rev Microbiol. 1981;35:405–452. doi: 10.1146/annurev.mi.35.100181.002201. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Cashel M., Lazzarini R. A., Kalbacher B. An improved method for thin-layer chromatography of nucleotide mixtures containing 32P-labelled orthophosphate. J Chromatogr. 1969 Mar 11;40(1):103–109. doi: 10.1016/s0021-9673(01)96624-5. [DOI] [PubMed] [Google Scholar]
- Cole M. A., Elkan G. H. Transmissible resistance to penicillin G, neomycin, and chloramphenicol in Rhizobium japonicum. Antimicrob Agents Chemother. 1973 Sep;4(3):248–253. doi: 10.1128/aac.4.3.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DOBROGOSZ W. J. THE INFLUENCE OF NITRATE AND NITRITE REDUCTION ON CATABOLITE REPRESSION IN ESCHERICHIA COLI. Biochim Biophys Acta. 1965 May 4;100:553–566. doi: 10.1016/0304-4165(65)90025-5. [DOI] [PubMed] [Google Scholar]
- Hanus F. J., Maier R. J., Evans H. J. Autotrophic growth of H2-uptake-positive strains of Rhizobium japonicum in an atmosphere supplied with hydrogen gas. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1788–1792. doi: 10.1073/pnas.76.4.1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laskey R. A., Mills A. D. Quantitative film detection of 3H and 14C in polyacrylamide gels by fluorography. Eur J Biochem. 1975 Aug 15;56(2):335–341. doi: 10.1111/j.1432-1033.1975.tb02238.x. [DOI] [PubMed] [Google Scholar]
- Lee J. H., Dobrogosz W. J. Effects of aerobic and anaerobic shock on catabolite repression in cyclic AMP suppressor mutants of Escherichia coli. J Bacteriol. 1983 May;154(2):992–994. doi: 10.1128/jb.154.2.992-994.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lepo J. E., Hanus F. J., Evans H. J. Chemoautotrophic growth of hydrogen-uptake-positive strains of Rhizobium japonicum. J Bacteriol. 1980 Feb;141(2):664–670. doi: 10.1128/jb.141.2.664-670.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim S. T., Shanmugam K. T. Regulation of hydrogen utilisation in Rhizobium japonicum by cyclic AMP. Biochim Biophys Acta. 1979 May 16;584(3):479–492. doi: 10.1016/0304-4165(79)90121-1. [DOI] [PubMed] [Google Scholar]
- Maier R. J., Campbell N. E., Hanus F. J., Simpson F. B., Russell S. A., Evans H. J. Expression of hydrogenase activity in free-living Rhizobium japonicum. Proc Natl Acad Sci U S A. 1978 Jul;75(7):3258–3262. doi: 10.1073/pnas.75.7.3258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maier R. J., Hanus F. J., Evans H. J. Regulation of hydrogenase in Rhizobium japonicum. J Bacteriol. 1979 Feb;137(2):825–829. doi: 10.1128/jb.137.2.825-829.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maier R. J., Merberg D. M. Rhizobium japonicum mutants that are hypersensitive to repression of H2 uptake by oxygen. J Bacteriol. 1982 Apr;150(1):161–167. doi: 10.1128/jb.150.1.161-167.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maier R. J., Mutaftschiev S. Reconstitution of H2 oxidation activity from H2 uptake-negative mutants of Rhizobium japonicum bacteroids. J Biol Chem. 1982 Feb 25;257(4):2092–2096. [PubMed] [Google Scholar]
- Maier R. J. Rhizobium japonicum mutant strains unable to grow chemoautotrophically with H2. J Bacteriol. 1981 Jan;145(1):533–540. doi: 10.1128/jb.145.1.533-540.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merberg D., Maier R. J. Mutants of Rhizobium japonicum with Increased Hydrogenase Activity. Science. 1983 Jun 3;220(4601):1064–1065. doi: 10.1126/science.220.4601.1064. [DOI] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Roberts G. P., Leps W. T., Silver L. E., Brill W. J. Use of two-dimensional polyacrylamide gel electrophoresis to identify and classify Rhizobium strains. Appl Environ Microbiol. 1980 Feb;39(2):414–422. doi: 10.1128/aem.39.2.414-422.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]