Abstract
We have previously shown that surfactant protein A (SP-A) mediates in vitro killing of mycoplasmas by alveolar macrophages (AMs) from resistant C57BL/6 mice through a nitric oxide (⋅NO)-dependent mechanism. Herein, SP-A-deficient [SP-A(−/−)] and inducible ⋅NO synthase-deficient [iNOS(−/−)] mice were infected intranasally with 105 or 107 colony-forming units of Mycoplasma pulmonis. SP-A(−/−) mice were as susceptible to mycoplasmal infection as highly susceptible C3H/He mice, and far more susceptible than resistant C57BL/6 mice. iNOS(−/−) mice had significantly greater numbers of mycoplasmas and severity of lung lesions than iNOS(+/+) controls. In vitro, AMs isolated from C57BL/6 mice, activated with IFN-γ, incubated with SP-A (25 μg/ml), and infected with 1010 colony-forming units of M. pulmonis, killed mycoplasmas within 6 h. Mycoplasmal killing was abrogated by 1,000 units/ml of copper-zinc superoxide dismutase. In the absence of AMs, incubation of M. pulmonis with the peroxynitrite generator 3-morpholinosynodiomine⋅HCl (SIN-1) effected complete killing of mycoplasmas by 90 min in a dose-dependent manner. Addition of copper-zinc superoxide dismutase (3,000 units/ml), which converts SIN-1 to a ⋅NO donor, prevented this killing. Neither of the reactive oxygen species generated by xanthine oxidase (10 milliunits/ml, plus 500 μM xanthine and 100 μM FeCl3), nor ⋅NO generated by 1-propanamine-3-(2-hydroxy-2-nitroso-1-propylhydrazine (PAPA NONOate) (100 μM) killed mycoplasmas. These data establish that peroxynitrite generation by AMs is necessary for the killing of a pathogen in vitro and in vivo.
Mycoplasma pneumoniae accounts for 20–30% of all pneumonias, causes illnesses such as tracheobronchitis, bronchiolitis, and pharyngitis (1), and exacerbates other respiratory disorders such as asthma (2) and chronic obstructive pulmonary disease (3). Furthermore, because of the wide diversity of clinical manifestations and the special testing required to identify active infections in different organs, it is becoming clear that M. pneumoniae diseases are greatly underdiagnosed (4). The mechanisms of defense against respiratory mycoplasmas are poorly understood, but current evidence suggests that innate immunity provides defense of the lungs while specific immunity defends against systemic dissemination of infection (5, 6).
Mycoplasma pulmonis infection in mice provides excellent in vivo models of human respiratory mycoplasmosis. Mouse strains differ markedly in resistance to M. pulmonis, with resistant C57BL/6 (C57BL) and susceptible C3H/He (C3H) mice representing the extremes in response to this infection. During the first 72 h postinfection (p.i.), the numbers of mycoplasmas decrease by >83% in the lungs of C57BL mice but increase by 18,000% in the lungs of C3H mice, a difference that cannot be explained by ciliary clearance (7) or specific antibody (6, 8).
Our studies of host defense against respiratory mycoplasmas have identified the alveolar macrophage (AM) as the primary effector cell in early mycoplasmal killing (9). In vitro studies indicated that activated AMs from resistant C57BL mice produced significant amounts of nitric oxide (⋅NO) and significantly decreased the number of colony-forming units (cfu) in the presence, but not in the absence, of the collectin surfactant protein A (SP-A). The importance of SP-A and ⋅NO in pulmonary defense has been established by others (10, 11).
Direct evidence that either SP-A or reactive oxygen–nitrogen intermediates contribute to M. pulmonis killing in vivo is lacking. In addition to ⋅NO, activated AMs are known to secrete a number of reactive oxygen species, including superoxide anions (O2⨪) (12), which can dismutate to form hydrogen peroxide (H2O2). O2⨪ and H2O2 might contribute to mycoplasmal killing directly, or by formation of other highly reactive oxygen-nitrogen intermediates such as hydroxyl radical (⋅OH) and peroxynitrite (ONOO−). Although the alveolar lining fluid contains a number of antioxidant substances (13, 14), recent in vivo evidence suggests that sufficient levels of ⋅NO and reactive oxygen–nitrogen intermediates remain to cause extensive damage to the alveolar epithelium and surfactant system (15). In addition, other pulmonary collectins, such as surfactant protein D may contribute to mycoplasmal defenses in vivo.
We designed a series of experiments to investigate the specific mechanism(s) involved in innate intrapulmonary mycoplasmal killing. First, SP-A [SP-A(−/−)] and iNOS [iNOS(−/−)] knockout mice were infected with M. pulmonis to determine the importance of SP-A and ⋅NO in mycoplasmal killing and lung lesion reduction in vivo. The roles of ⋅NO, O2⨪ and ONOO− in SP-A mediated mycoplasmal killing by AMs was further characterized in vitro by using AMs from mycoplasma resistant C57BL mice (16). Finally, the mycoplasmacidal activities of O2⨪, ⋅NO, ONOO−, H2O2, and ⋅OH were tested with the aid of an AM-free system and generators of specific chemical reactive species. The results indicate that SP-A-mediated AM production of ONOO− is necessary for the killing of an important respiratory pathogen in vivo and in vitro.
MATERIALS AND METHODS
Media and Chemicals.
PBS and DMEM were from Mediatech (Herndon, VA). Saline was obtained from Abbott. BBL Mycoplasma broth base was obtained from Becton Dickinson. 3-Morpholinosynodiomine⋅HCl (SIN-1), bovine copper–zinc superoxide dismutase (Cu, ZnSOD), xanthine oxidase (XO), and 1-propanamine-3-(2-hydroxy-2-nitroso-1-propylhydrazine) (PAPA NONOate) were from Calbiochem. Catalase was from Worthington. Dihydrorhodamine 123 came from Molecular Probes. Diff Quik stain kits were from Baxter Healthcare (Mundelein, IL), and all other chemicals, unless specified, were from Sigma.
Purification of SP-A.
SP-A was purified sterilely from the bronchoalveolar lavage (BAL) fluid of patients with alveolar proteinosis as described (17). Polyacrylamide gel electrophoresis and Western blot analysis indicated that SP-A was free of albumin, Ig, and surfactant protein D. SP-A was tested at the University of Alabama at Birmingham (UAB) Media Preparation Shared Facility and found to contain ≤2 pg of endotoxin per 25 μg/ml SP-A. Aliquots were cultured for aerobic bacteria in BBL brain heart infusion broth (Becton Dickinson), and only culture-negative SP-A was used in experiments.
Animals.
C57BL (C57BL/6NCr) and C3H (C3H/HeNCr) mice were from the Frederick Cancer Research and Development Center, National Cancer Institute, and used in studies at 8–12 weeks of age. C57BL [C57BL/6J-Nos2tm1Lau (N7F4)] transgenic mice lacking inducible nitric oxide synthase [iNOS(−/−)] and 129/J mice were obtained from The Jackson Laboratory. Breeding pairs of 129/Ola X Black Swiss SP-A-deficient [SP-A(−/−)] mice were provided by J. Whitsett and T. Korfhagen (University of Cincinnati) and were bred in Trexler-type isolators (19). All mice were monitored by J.R.L. and found to be negative for the presence of murine pathogens (20) except for the SP-A(−/−) mice (see Results). Mice were anesthetized for in vivo inoculation and for euthanasia by injection with ketamine (Aveco, Fort Dodge, IA) and xylazine (Haver, Shawnee, KS) (6).
Mycoplasmas.
The UAB CT strain of M. pulmonis was used in all experiments (21). For in vivo experiments, 3 × 107 cfu per ml stock was diluted in broth A (21) to 105 or 107 cfu per 50 μl volumes. Inoculations were given intranasally with control mice receiving broth A alone. cfu in all inocula were confirmed by enumeration after serial dilution and growth on agar plates (22). For in vitro experiments, mycoplasmas were incubated at 37°C for 18 h before use to ensure active growth in the logarithmic phase.
Quantitative Lung Cultures.
Whole lungs were aseptically removed at 1, 2, 3, or 7 days, individually minced, and sonicated for 30 s in broth A. Ten-fold dilutions of each lung homogenate were made in 24-well plates and enumerated by culture on agar plates (22).
Assessment of Lesion Severity.
Lung sections were coded randomly and scored subjectively for lesion severity on the basis of characteristic lesions of respiratory mycoplasmosis: (i) exudate in airway lumina, (ii) hyperplasia-dysplasia of mucosal epithelium, (iii) peribronchial and perivascular lymphoid accumulation, and (iv) inflammatory infiltration in alveoli (6).
Macrophage Isolation.
BALs were performed as described (16). Cells were >90% viable by trypan blue exclusion and contained >95% macrophages as identified on cytospin with Diff Quik stain.
Nitrotyrosine Immunohistochemistry.
Paraffin-embedded lung sections from 3-day-infected C57BL iNOS(+/+) and C57BL iNOS(−/−) mice were stained for nitrotyrosine [antibody provided by J.S. Beckman and Y.Z. Ye, UAB (23)], or iNOS (Transduction Laboratories, Lexington, KY) as described (15). BALs were performed on 3-day-infected C57BL iNOS(+/+) mice and C57BL iNOS(−/−) mice for iNOS staining. Cells were plated onto Lab-Tek chamber slides (Nunc), incubated for 1 h, washed in PBS, and fixed in 4% paraformaldehyde. AMs were washed, permeabilized, and stained for iNOS protein as for tissues.
Mycoplasmal Killing in Vitro.
AMs (1 × 105) were used in in vitro assays as described (8, 16, 24–26). To determine the role of O2⨪ in SP-A-mediated mycoplasmal killing, 1,000 units/ml (286 μg/ml) of Cu, ZnSOD were added to all incubation medias before addition of SP-A and maintained throughout each experiment. Control samples without Cu, ZnSOD were run simultaneously. A combination of catalase (500 units/ml, 5,000 units/ml, or 10,000 units/ml) and Cu, ZnSOD (1,000 units/ml) was added to some cell cultures before addition of SP-A.
Generation of ⋅NO and O2⨪.
Exposure of mycoplasmas (1012) to SIN-1 (1 mM or 200 μM) was performed in autoclaved 130-ml centrifuge tubes in 10 ml of 25 mM Hepes buffer. Tubes were agitated in a shaking water bath at 37°C (27). Aliquots were taken at 0, 20, 45, 60, and 90 min for determination of cfu (22). The generation of ONOO− by SIN-1 was calculated from the oxidation of dihydrorhodamine 123 (18, 27, 28). SIN-1C was generated by allowing SIN-1 to decompose in Hepes buffer. ⋅NO was generated by PAPA in 25 mM Hepes buffer, pH 7.4. ⋅NO concentration was measured with an ISO–NO electrochemical probe (World Precision Instruments, Sarasota, FL) (29). Exposure to mycoplasmas was performed as described for SIN-1 exposures.
Xanthine/XO Incubations.
Reactive oxygen species were generated by 10 milliunits/ml XO, 500 μM xanthine plus 100 μM FeCl3 (10 mM) and EDTA (10 mM) in 25 mM Hepes buffer at 37°C, pH 7.4 (30). Exposure of mycoplasmas to xanthine/XO was performed as described for SIN-1 exposures. Based on the calculated depletion of the substrate, some samples received additional xanthine every 15 min to maintain constant substrate levels (30).
Statistics.
All experiments had a minimum of six mice per group for in vivo infection studies or four replicates per group for in vitro studies. Each experiment was repeated twice to assure reproducibility. Data were analyzed by using ANOVA followed by Tukey’s multigroup comparison of means for parametric data and by the Kruskal–Wallis ANOVA and Pearson’s multigroup comparison of the means for nonparametric data (31). Mycoplasma cfu were converted to common logarithms for statistical analysis and results were expressed as means ± SE. P ≤ 0.05 was considered significant.
RESULTS
Involvement of SP-A in Mycoplasmal Killing in Vivo.
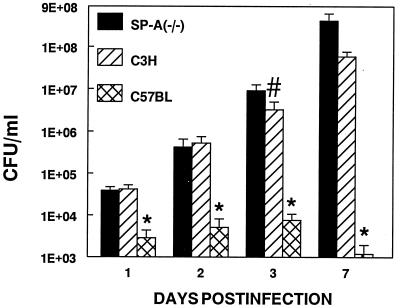
We infected SP-A(−/−) transgenic mice with 105 cfu (32) of M. pulmonis and assessed mycoplasma numbers in whole-lung homogenates and lung lesion severity at 1, 2, 3, or 7 days p.i. SP-A(−/−) mice were infected concurrently with susceptible C3H and resistant C57BL mice. SP-A(−/−) mice had significantly more recoverable mycoplasmas in their lungs than the resistant C57BL mice at all time points and significantly more recoverable mycoplasmas than susceptible C3H mice at 3 days p.i. (Fig. 1). Specific antibody against mycoplasmas is detectable by 3 days p.i. and does not effect mycoplasmal clearance in susceptible animals (6, 33). Histopathology of mycoplasma-infected lungs demonstrated no significant differences in lung pathology between the SP-A(−/−) mice and the mycoplasma-susceptible C3H mice; however, both mouse strains had significantly higher lung lesion scores than mycoplasma-resistant C57BL mice at all time points for all lesion parameters (data not shown).
Figure 1.
Transgenic SP-A(−/−) mice, mycoplasma-resistant C57BL, and susceptible C3H mice were infected intranasally with 105 cfu of M. pulmonis. Mice were euthanized at 1, 2, 3, or 7 days p.i., and cfu numbers (total recoverable mycoplasmas) were determined on whole-lung homogenates. cfu are graphed on a logarithmic scale. ∗, significant difference from the other two remaining groups at each time point, P < 0.05, #, significant difference from the other two groups at this time point, P < 0.05. Results of quantitative cultures are mean ± SE; n ≥18.
Age-matched SP-A(−/−) and 129/J mice were infected intranasally with 105 M. pulmonis, and mycoplasma numbers were assessed in whole-lung homogenates at 1, 2, 3, or 7 days p.i. SP-A(−/−) mice had higher cfu counts at all time points and significantly higher cfu counts at 3 days p.i. (data not shown). Inbred 129/J mice were selected for this study as representative parental strain controls for SP-A(−/−) mice (34). Black Swiss mice were not used because of their outbred status.
SP-A(−/−) mice were tested extensively for murine pathogens and found to be negative (20) except for mRNA of the intestinal pathogen Helicobacter hepaticus (J. Fox, Massachusetts Institute of Technology) by using PCR. Histopathologic sections were made of the liver and large intestine of all SP-A(−/−) mice used in infection studies and none had lesions consistent with H. hepaticus disease (35). Although H. hepaticus may alter T cell function in healthy animals (36), T cells have not been found to play a significant role in early mycoplasmal clearance (5).
Involvement of ⋅NO in Mycoplasmal Killing in Vivo.
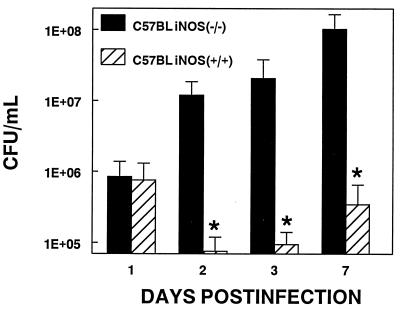
We infected C57BL iNOS(−/−) and C57BL iNOS(+/+) mice with 1.5 × 107 cfu (32) of M. pulmonis and quantified mycoplasma cfu in whole-lung homogenates and severity of lung lesions at 1, 2, 3, or 7 days p.i. Significantly more mycoplasmas were recovered from the lungs of C57BL iNOS(−/−) than C57BL iNOS(+/+) at every time point after day 1 p.i. (Fig. 2). Histopathology of mycoplasma-infected lungs demonstrated significantly higher lesion indices for C57BL iNOS(−/−) than C57BL iNOS(+/+) for all lesion parameters at 3 days p.i. (data not shown).
Figure 2.
C57BL iNOS(−/−) and control C57BL iNOS(+/+) mice were infected intranasally with 1.5 × 107 cfu per ml M. pulmonis. Mice were euthanized at 1, 2, 3, or 7 days p.i., and cfu numbers (total recoverable mycoplasmas) were determined on whole-lung homogenates. ∗, significant difference between control and experimental conditions at each time point, P < 0.05. Results of quantitative cultures are mean ± SE; n ≥ 18.
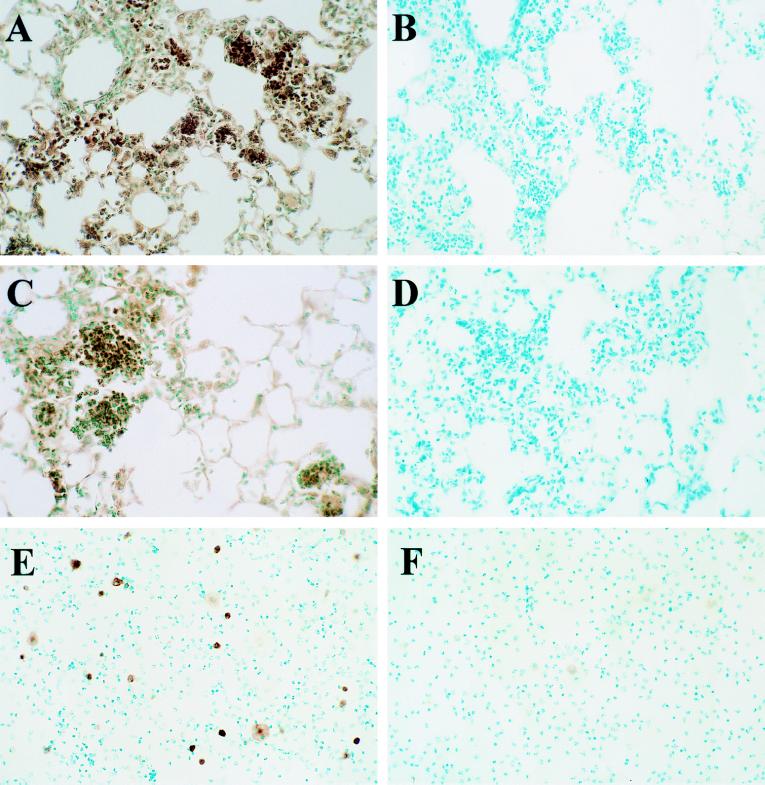
Immunohistochemical staining of the lungs of control C57BL iNOS(+/+) demonstrated significant nitrotyrosine mainly in areas of neutrophilic inflammation, and similar amounts of nitrotyrosine staining in the lungs of C57BL iNOS(−/−) mice (Figs. 3 A–D). Immunostaining for iNOS protein in C57BL iNOS(+/+) controls showed strongly positive cytoplasmic staining of AMs both in lung sections and cells isolated by BAL. In contrast, no iNOS positive cells were identified in lungs or BAL from C57BL iNOS(−/−) mice (Fig. 3 E–F).
Figure 3.
Visualization of nitrotyrosine residues and iNOS protein in the lungs of transgenic C57BL iNOS(−/−) and control C57BL iNOS(+/+) mice infected 3 days with 1.5 × 107 cfu per ml M. pulmonis. (A) Nitrotyrosine staining of lungs from resistant C57BL iNOS(+/+) mice. (B) Nitrotyrosine staining from A in the presence of excess nitrotyrosine (10 mM). (C) Nitrotyrosine staining of lungs of C57BL iNOS(−/−) mice. (D) Nitrotyrosine staining from C in the presence of excess nitrotyrosine (10 mM). (E) iNOS staining of BALs from C57BL iNOS(+/+) mice. (F) iNOS staining of BALs C57BL iNOS(−/−) mice. Shown are representative pictures of results, which were reproduced at least twice.
Involvement of O2⨪ and SP-A in Mycoplasmal Killing by AMs.
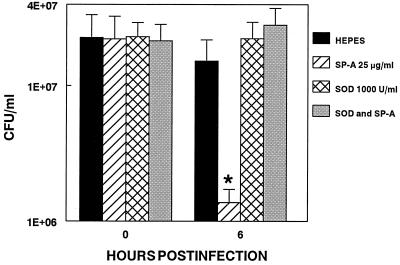
Preincubation of IFN-γ activated AMs from mycoplasma resistant C57BL mice with SP-A significantly enhanced the killing of mycoplasmas within 6 h p.i. (P < 0.001). The addition of 1,000 units/ml of Cu, ZnSOD reversed this SP-A mediated killing (Fig. 4). Cu, ZnSOD in combination with catalase (500–10,000 units/ml) had no effect on mycoplasmal killing (data not shown). In the absence of AMs, neither catalase nor Cu, ZnSOD effected mycoplasmal growth (data not shown).
Figure 4.
AMs (1 × 105) were activated with 100 units/ml IFN-γ, washed, and incubated with 1,000 units/ml of Cu, ZnSOD. AMs were treated with SP-A (25 μg/ml) or Hepes (5 mM), infected with 1010 cfu of M. pulmonis, and incubated for 0 and 6 h. Results of quantitative cultures are mean ± SE from a total of three experiments with 12–15 data points per group. ∗, significant difference between control and experimental groups at this time point, P <0.05.
Involvement of O2⨪ and ⋅NO in Mycoplasmal Killing in the Absence of AMs.
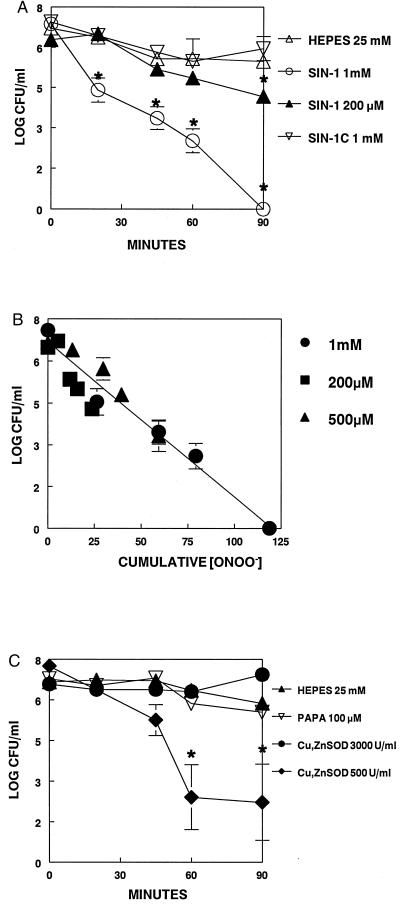
SIN-1 (1 mM) decreased mycoplasma cfu significantly by 20 min and completely killed all mycoplasmas by 90 min. This concentration of SIN-1 generated ≈1.0 μM/min (1.32 ± 0.38, n = 5) ONOO− at 37°C. SIN-1 (500 μM) caused significant mycoplasmal killing by 45 min (22 μM ONOO−) and SIN-1 (200 μM) caused significant killing by 90 min (18 μM ONOO−; Fig. 5A). Killing of mycoplasmas depended on the cumulative concentration of ONOO− released by SIN-1 because significant reduction of mycoplasmal numbers occurred only after exposure to ≈20 μM of ONOO− (Fig. 5B). SIN-1C, the inactive decomposition product of SIN-1, had no effect on mycoplasma cfu counts. Cu, ZnSOD (3,000 units/ml) decreased ONOO− generation by >93%, thus converting SIN-1 to a ⋅NO donor and protecting against the mycoplasmacidal effects of SIN-1. Cu, ZnSOD (500 units/ml) decreased ONOO− generation by 50–70% and protected against SIN-1 toxicity proportionately (Fig. 5C). PAPA (100 μM) generated ≈4–6 μM ⋅NO and had no effect on mycoplasmal growth up to 90 min.
Figure 5.
M. pulmonis was grown to late logarithmic phase, washed to remove serum, and resuspended in 10 ml of 25 mM Hepes buffer, pH 7.4. Aliquots were taken at 0, 20, 45, 60, and 90 min for determination of cfu. (A) Hepes 25 mM, mycoplasmas alone; SIN-1 1 mM, mycoplasmas + 1 mM SIN-1; SIN-1 200 μM, mycoplasmas + 200 μM SIN-1; SIN-1C, mycoplasmas + 1 mM SIN-1C. (B) cfu vs. ONOO− concentration for the indicated concentrations of SIN-1 as measured by dihydrorhodamine 123 oxidation. (C) Hepes 25 mM, mycoplasmas alone; PAPA 100 μM, mycoplasmas +100 μM PAPANONOate; Cu, ZnSOD 3,000 units/ml, mycoplasmas +1 mM SIN-1 + 3,000 units/ml Cu, ZnSOD.;Cu, ZnSOD 500 units/ml, mycoplasmas +1 mM SIN-1 + 500 units/ml Cu, ZnSOD. ∗, significant difference between control and experimental groups at each time point, P <0.05.
Lack of Involvement of Hydrogen Peroxide (H2O2), Hydroxyl Radical (⋅OH), and Superoxide (O2⨪) in Mycoplasmal Killing in the Absence of AMs.
At pH 7.4, >70% of the reactive oxygen species generated by the action of XO on xanthine are in the form of H2O2, with the remaining 30% appearing as O2⨪ (30). In the presence of 500 μM xanthine and 10 milliunits/ml XO, ≈300 μM H2O2 is produced (30). In the presence of iron, O2⨪ reduces ferric iron (Fe3+), which in turn reduces H2O2 to form the highly toxic ⋅OH (37). The generation of H2O2 or ⋅OH had minimal effect on mycoplasmal growth (data not shown).
DISCUSSION
The purpose of this study was to identify the essential mechanism(s) of SP-A-mediated antimycoplasmal defense in the lungs, particularly the role(s) of reactive oxygen–nitrogen intermediates. We utilized transgenic mice to confirm the importance of SP-A and ⋅NO in resistance against mycoplasmal infections in vivo. Transgenic mice lacking the functional SP-A gene have been shown to be more susceptible to group B streptococci (38), Staphylococcus aureus, and Pseudomonas aeruginosa (39). C3H mice have been found to be among the most highly susceptible mouse strains to mycoplasmal infections in comparison to 16 other strains (7, 32, 40) and therefore, represent the extreme in susceptibility to this bacteria. The SP-A(−/−) mice were on a mixed genetic background and therefore, not suitable for direct matched strain comparisons. We should emphasize that our intention was to compare the response of SP-A(−/−) mice to mycoplasmas with the responses of SP-A(+/+) resistant C57BL and susceptible C3H mice. SP-A(−/−) mice were found to be equally or more susceptible to mycoplasmal lung infection as the highly susceptible C3H mice. SP-A(−/−) mice also were more susceptible to mycoplasmal infections than their original 129/J parental strain. Taken together, these in vivo data give strong evidence that SP-A plays an important role in lung mycoplasmal killing. Because SP-A(−/−) mice contain normal amounts of SP-D, this is the first indication that SP-D is not of primary importance for resistance to mycoplasmas in vivo.
C57BL iNOS(−/−) mice were unable to clear mycoplasmas as efficiently as control C57BL iNOS(+/+) mice and had significantly higher lung lesion indices for all histopathologic parameters at 3 days p.i. There was no significant difference in lesion indices between the two mouse strains at 7 days p.i. which may reflect the appearance of specific antibody. Immunostaining for nitrotyrosine in the lungs of infected control C57BL iNOS(+/+) and C57BL iNOS(−/−) demonstrated considerable amounts of nitrotyrosine in both strains, whereas staining for iNOS protein was positive only in AMs of C57BL iNOS(+/+) mice. Significant nitrotyrosine in lungs of C57BL iNOS(−/−) mice may be explained by the fact that these mice have normal amounts of eNOS and bNOS that are capable of producing ⋅NO within the lung. Nitrotyrosine was detected mainly in areas of neutrophilic inflammation; hypochlorous acid produced by neutrophils may react with nitrite (the stable breakdown product of ⋅NO) to form reactive intermediates that are capable of nitrating tyrosine (41). These data suggest that ⋅NO produced by AMs is essential for mycoplasmal killing and deficiency of the iNOS protein cannot be compensated for by ⋅NO from other sources. In contrast, indiscriminate production of ONOO− by lung cells can result in significant injury, as evidenced by increased lung pathology in the C57BL iNOS(−/−) mice at 3 days p.i. These data emphasize the dual nature of ONOO− as a protective and destructive agent.
Previously, we showed that mycoplasmal killing in vitro requires (i) the presence of SP-A and (ii) the generation of ⋅NO and/or its toxic metabolites (16). SP-A is known to effect release of both reactive oxygen (42, 43) and nitrogen (44) species, and these compounds may work singly or in concert to cause bacterial killing. Increased production of ⋅NO has been linked with the microbicidal activity of AMs, however, ⋅NO alone does not appear to be directly toxic to bacteria (27, 45). ONOO−, a strong oxidant formed as a reaction product of O2⨪ and ⋅NO, has been shown to be highly bactericidal (27, 46). We found that 105 IFN-γ activated AMs, when infected with mycoplasmas, produced 0.6 μM⋅h−1 of ⋅NO and, although this was a significant amount of ⋅NO, there was no mycoplasmal killing. In the presence of SP-A, activated AMs produced ≈45% more ⋅NO (1.1 μM⋅h−1) and caused a significant decrease in mycoplasmal cfu numbers. The dependence of this killing mechanism on ⋅NO was confirmed by the addition of NG-monomethyl-l-arginine, which abrogated SP-A-mediated killing (16). In the present study, we found that the addition of Cu, ZnSOD also attenuated SP-A mediated mycoplasmal killing by activated AMs, implicating ONOO− as the toxic oxygen–nitrogen intermediate. Catalase had no effect on SP-A-mediated mycoplasmal killing, indicating that H2O2 was not important.
In the absence of AMs, we found that ⋅NO had no effect on mycoplasmal survival, whereas the combination of ⋅NO and O2⨪ (generated by SIN-1) was toxic. Exposure of mycoplasmas to 1 mM SIN-1 caused a significant decrease in mycoplasmas by 20 min and correlated with ONOO− concentration. Cu, ZnSOD was protective against SIN-1 toxicity only after >90% of ONOO− production was inhibited.
These data stress the importance of the AM in pulmonary antimycoplasmal defenses. Even if all of the ⋅NO produced by activated AMs in our system was completely converted to ONOO− (47), we could expect only 67% less ONOO− formation than required for mycoplasmal killing in an AM-free system. Calculations of ONOO− formation by AMs (47) and the size of the phagolysosome suggest that concentrations of ONOO− within this compartment may be as high as 500 μM (48). These data suggest that mycoplasmal killing by AMs occurs within the phagolysosome.
We have demonstrated that SP-A is an essential component of AM-mediated mycoplasmal killing in vitro as well as for host resistance in vivo. Despite reported resistance of mycoplasmas to reactive oxygen species (49), we found that ⋅NO and O2⨪ produced by AMs was a crucial factor in mycoplasmal resistance both in vivo and in vitro (16). These data indicate that SP-A-mediated killing of M. pulmonis by activated AMs occurs via the production of ONOO−.
Acknowledgments
We thank Dr. J. Crow for expert advice; Dr. D. Shaw for endotoxin testing; Drs. J. Whitsett and T. Korfhagen for breeding pairs of SP-A knockout mice, and K. Hardiman, J. Hosmer, M. Phillips, and M. Shackelford for technical support. This work was supported by National Institutes of Health Grants RR-1105 (to J.R.L.), HL31197 and HL51173 (to S.M.), funds from the Veterans Affairs Research Service (to J.R.L.), and Grant N00014-1-0309 from the Office of Naval Research (to S.M.). J.M.H.-D. is a Parker B. Francis Fellow.
ABBREVIATIONS
- SP-A
surfactant protein A
- AM
alveolar macrophage
- Cu
ZnSOD, copper–zinc superoxide dismutase
- SIN-1
3-morpholinosynodiomine⋅HCl
- p.i.
postinfection
- XO
xanthine oxidase
- BAL
branchoalveolar lavage
References
- 1.Cassell G H, Gray G C, Waites K B. In: Harrison’s Principles of Internal Medicine. Fauci, Pack E, editors. New York: McGraw–Hill; 1997. pp. 1–29. [Google Scholar]
- 2.Gil J C, Cedillo R C, Mayagoitia B G, Paz M D. Ann Allergy. 1993;70:23–25. [PubMed] [Google Scholar]
- 3.Melbye H, Kongerud J, Vorland L. Eur Respir J. 1994;7:1239–1245. doi: 10.1183/09031936.94.07071239. [DOI] [PubMed] [Google Scholar]
- 4.Cassell G H. West J Med. 1995;162:172–175. [PMC free article] [PubMed] [Google Scholar]
- 5.Cartner S C, Lindsey J R, Gibbs-Erwin J, Cassell G H, Simecka J W. Infect Immun. 1998;66:3485–3491. doi: 10.1128/iai.66.8.3485-3491.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cartner S C, Simecka J R, Lindsey J R, Cassell G H, Davis J K. Infect Immun. 1995;63:4138–4142. doi: 10.1128/iai.63.10.4138-4142.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Parker R F, Davis J K, Blalock D K, Thorp R B, Simecka J W, Cassell G H. Infect Immun. 1987;55:2631–2635. doi: 10.1128/iai.55.11.2631-2635.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Davis J K, Davidson M K, Schoeb T R, Lindsey J R. Am Rev Respir Dis. 1992;145:406–411. doi: 10.1164/ajrccm/145.2_Pt_1.406. [DOI] [PubMed] [Google Scholar]
- 9.Hickman-Davis J M, Michalek S M, Gibbs-Erwin J, Lindsey J R. Infect Immun. 1997;65:2278–2282. doi: 10.1128/iai.65.6.2278-2282.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nathan C. J Clin Invest. 1997;100:2417–2423. doi: 10.1172/JCI119782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wright J R. Physiol Rev. 1997;77:931–962. doi: 10.1152/physrev.1997.77.4.931. [DOI] [PubMed] [Google Scholar]
- 12.Bastian N R, Hibbs J B., Jr Opin Immunol. 1994;6:131–139. doi: 10.1016/0952-7915(94)90044-2. [DOI] [PubMed] [Google Scholar]
- 13.Cantin A M, North S L, Hubbard R C, Crystal R G. J Appl Physiol. 1987;63:152–157. doi: 10.1152/jappl.1987.63.1.152. [DOI] [PubMed] [Google Scholar]
- 14.Matalon S, Holm B A, Baker R R, Whtifield M K, Freeman B A. Biochem Biophys Acta. 1990;1035:121–127. doi: 10.1016/0304-4165(90)90105-6. [DOI] [PubMed] [Google Scholar]
- 15.Haddad I Y, Pataki G, Hu P, Galliani C, Beckman J S, Matalon S. J Clin Invest. 1994;94:2407–2413. doi: 10.1172/JCI117607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hickman-Davis J M, Lindsey J R, Zhu S, Matalon S. Am J Physiol. 1998;274:L270–L277. doi: 10.1152/ajplung.1998.274.2.L270. [DOI] [PubMed] [Google Scholar]
- 17.Haddad I Y, Ischiropoulos H, Holm B A, Beckman J S, Baker J R, Matalon S. Am J Physiol. 1993;265:L555–L564. doi: 10.1152/ajplung.1993.265.6.L555. [DOI] [PubMed] [Google Scholar]
- 18.Haddad I Y, Zhu S, Ischiropoulos H, Matalon S. Am J Physiol. 1996;270:L281–L288. doi: 10.1152/ajplung.1996.270.2.L281. [DOI] [PubMed] [Google Scholar]
- 19.Trexler P C. In: The Mouse in Biomedical Research, Normative Biology, Immunology, and Husbandry. Foster H L, Small J D, Fox J G, editors. Vol. 3. New York: Academic; 1983. pp. 1–16. [Google Scholar]
- 20.Faulkner C B, Simecka J W, Davidson M K, Davis J K, Schoeb T R, Lindsey J R, Everson M P. Infect Immun. 1995;63:4084–4090. doi: 10.1128/iai.63.10.4084-4090.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Davidson M K, Lindsey J R, Parker R F, Tully J G, Cassell G H. Infect Immun. 1988;56:2156–2162. doi: 10.1128/iai.56.8.2156-2162.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Davis J K, Delozier K M, Asa D K, Minion F C, Cassell G H. Infect Immun. 1980;29:590–599. doi: 10.1128/iai.29.2.590-599.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Beckman J S, Ye Y Z, Anderson P G, Chen J, Accavitti M A, Tarpey M M, White C R. Biol Chem Hoppe-Seyler. 1994;375:81–88. doi: 10.1515/bchm3.1994.375.2.81. [DOI] [PubMed] [Google Scholar]
- 24.McNeely T B, Coonrod J D. J Infect Dis. 1993;167:91–97. doi: 10.1093/infdis/167.1.91. [DOI] [PubMed] [Google Scholar]
- 25.Downing J F, Pasula R, Wright J R, Twigg H L, Martin I. Proc Natl Acad Sci USA. 1995;92:4848–4852. doi: 10.1073/pnas.92.11.4848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Davis J K, Davidson M, Schoeb T R. Investigators’ Report 47. Cambridge, MA: Health Effects Institute; 1991. [PubMed] [Google Scholar]
- 27.Brunelli L, Crow J P, Beckman J S. Arch Biochem Biophys. 1995;316:327–334. doi: 10.1006/abbi.1995.1044. [DOI] [PubMed] [Google Scholar]
- 28.Kooy N W, Royall J A, Ischiropoulos H, Beckman J S. Free Rad Biol Med. 1994;16:149–156. doi: 10.1016/0891-5849(94)90138-4. [DOI] [PubMed] [Google Scholar]
- 29.Guo Y, DuVall M D, Crow J P, Matalon S. Am J Physiol. 1998;274:L369–L377. doi: 10.1152/ajplung.1998.274.3.L369. [DOI] [PubMed] [Google Scholar]
- 30.Engstrom P C, Easterling L, Baker R R, Matalon S. J Appl Physiol. 1990;69:2078–2084. doi: 10.1152/jappl.1990.69.6.2078. [DOI] [PubMed] [Google Scholar]
- 31.Siegel J. statistix. Tallahassee, FL: Analytical Software; 1994. [Google Scholar]
- 32.Davis J K, Parker R F, White H, Dziedzic D, Taylor G, Davidson M K, Cox N R, Cassell G H. Infect Immun. 1985;50:647–654. doi: 10.1128/iai.50.3.647-654.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Simecka J W, Davis J K, Cassell G H. Infect Immun. 1989;57:3570–3575. doi: 10.1128/iai.57.11.3570-3575.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Simpson E M, Linder C C, Sargent E E, Davisson M T, Mobraaten L E, Sharp J J. Nat Genet. 1997;16:19–27. doi: 10.1038/ng0597-19. [DOI] [PubMed] [Google Scholar]
- 35.Fox J G, Dewhirst F E, Tully J G, Paster B J, Yan L, Taylor N S, Jr, M. J. C, Gorelick P L, Ward J M. J Clin Microbiol. 1994;32:1238–1245. doi: 10.1128/jcm.32.5.1238-1245.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Whary M T, Morgan T J, Dangler C A, Gaudes K J, Taylor N S, Fox J G. Infect Immun. 1998;66:3142–3148. doi: 10.1128/iai.66.7.3142-3148.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Freeman B A, Crapo J D. Lab Invest. 1982;47:412–426. [PubMed] [Google Scholar]
- 38.LeVine A M, Bruno M D, Huelsman K M, Ross G F, Whitsett J A, Korfhagen T R. J Immunol. 1997;158:4336–4340. [PubMed] [Google Scholar]
- 39.Crouch E C. Am J Respir Cell Mol Biol. 1998;19:177–201. doi: 10.1165/ajrcmb.19.2.140. [DOI] [PubMed] [Google Scholar]
- 40.Cartner S C, Simecka J W, Briles D E, Cassell G H, Lindsey J R. Infect Immun. 1996;64:5326–5331. doi: 10.1128/iai.64.12.5326-5331.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Eiserich J P, Hristova M, Cross C E, Jones A D, Freeman B A, Halliwell B, van der Vliet A. Nature (London) 1998;391:393–397. doi: 10.1038/34923. [DOI] [PubMed] [Google Scholar]
- 42.Van Iwaarden F, Welmers B, Verhoef J, Haagsman H P, VanGolde L M G. Am J Respir Cell Mol Biol. 1990;2:91–98. doi: 10.1165/ajrcmb/2.1.91. [DOI] [PubMed] [Google Scholar]
- 43.Weissbach S, Neundank A, Pettersson M, Schaberg T, Pison U. Am J Physiol. 1994;267:L660–L666. doi: 10.1152/ajplung.1994.267.6.L660. [DOI] [PubMed] [Google Scholar]
- 44.Blau H, Riklis S, Van Iwaarden J F, McCormack F X, Kalina M. Am J Physiol. 1997;272:L1198–L1204. doi: 10.1152/ajplung.1997.272.6.L1198. [DOI] [PubMed] [Google Scholar]
- 45.Pacelli R, Wink D A, Cook J A, Krishna M C, DeGraff W, Friedman N, Tsokos M, Samuni A, Mitchell J B. J Exp Med. 1995;182:1469–1479. doi: 10.1084/jem.182.5.1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhu L, Gunn C, Beckman J S. Arch Biochem Biophys. 1992;298:452–457. doi: 10.1016/0003-9861(92)90434-x. [DOI] [PubMed] [Google Scholar]
- 47.Ischiropoulos H, Zhu L, Beckman J S. Arch Biochem Biophys. 1992;298:446–451. doi: 10.1016/0003-9861(92)90433-w. [DOI] [PubMed] [Google Scholar]
- 48.Denicola A, Rubbo H, Rodriguez D, Radi R. Arch Biochem Biophys. 1993;304:279–286. doi: 10.1006/abbi.1993.1350. [DOI] [PubMed] [Google Scholar]
- 49.Meier B, Habermehl G G. Arch Biochem Biophys. 1990;277:74–79. doi: 10.1016/0003-9861(90)90552-a. [DOI] [PubMed] [Google Scholar]