Abstract
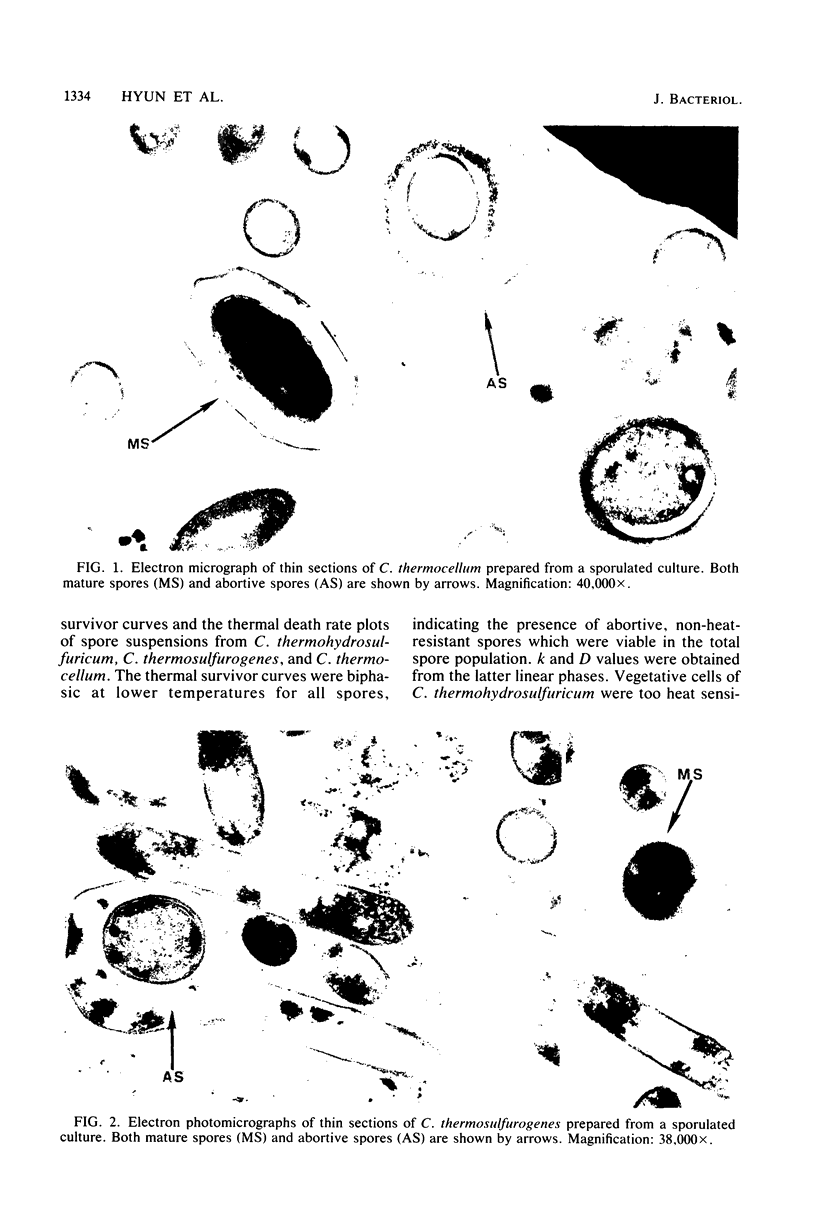
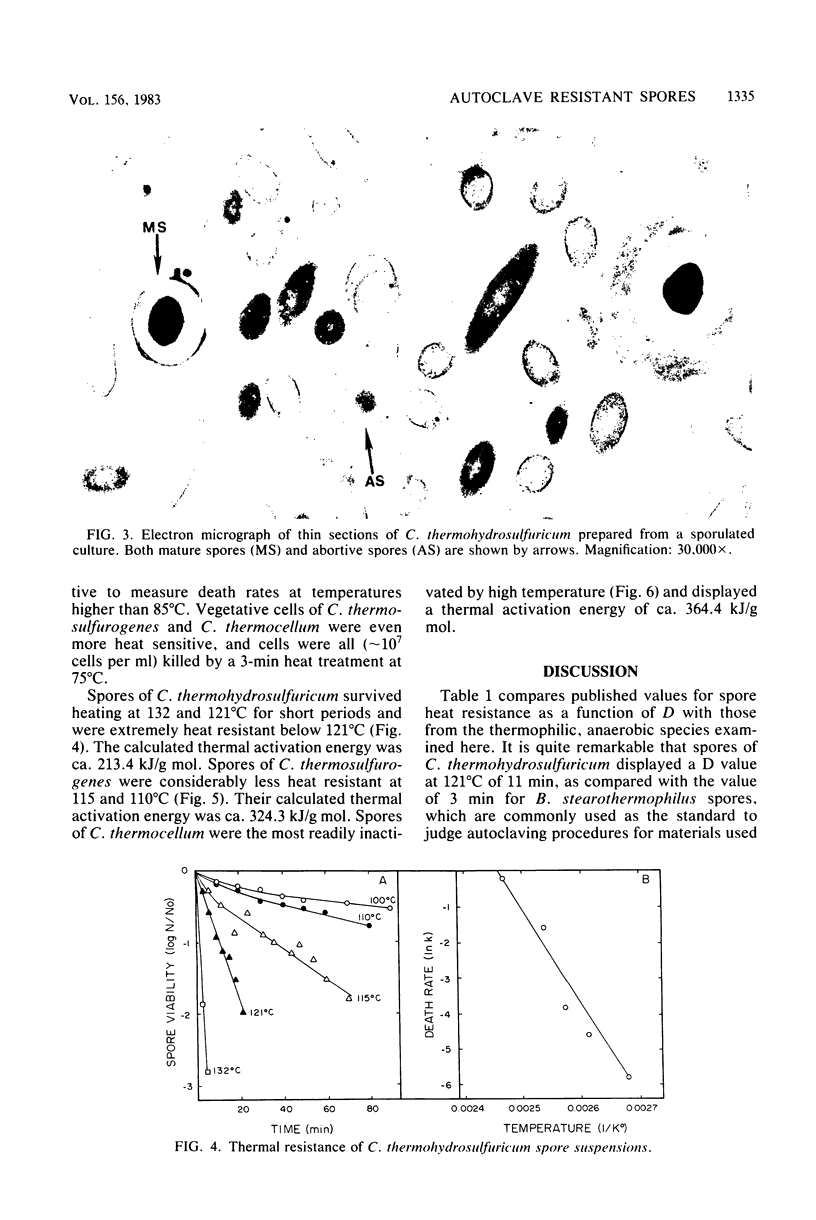
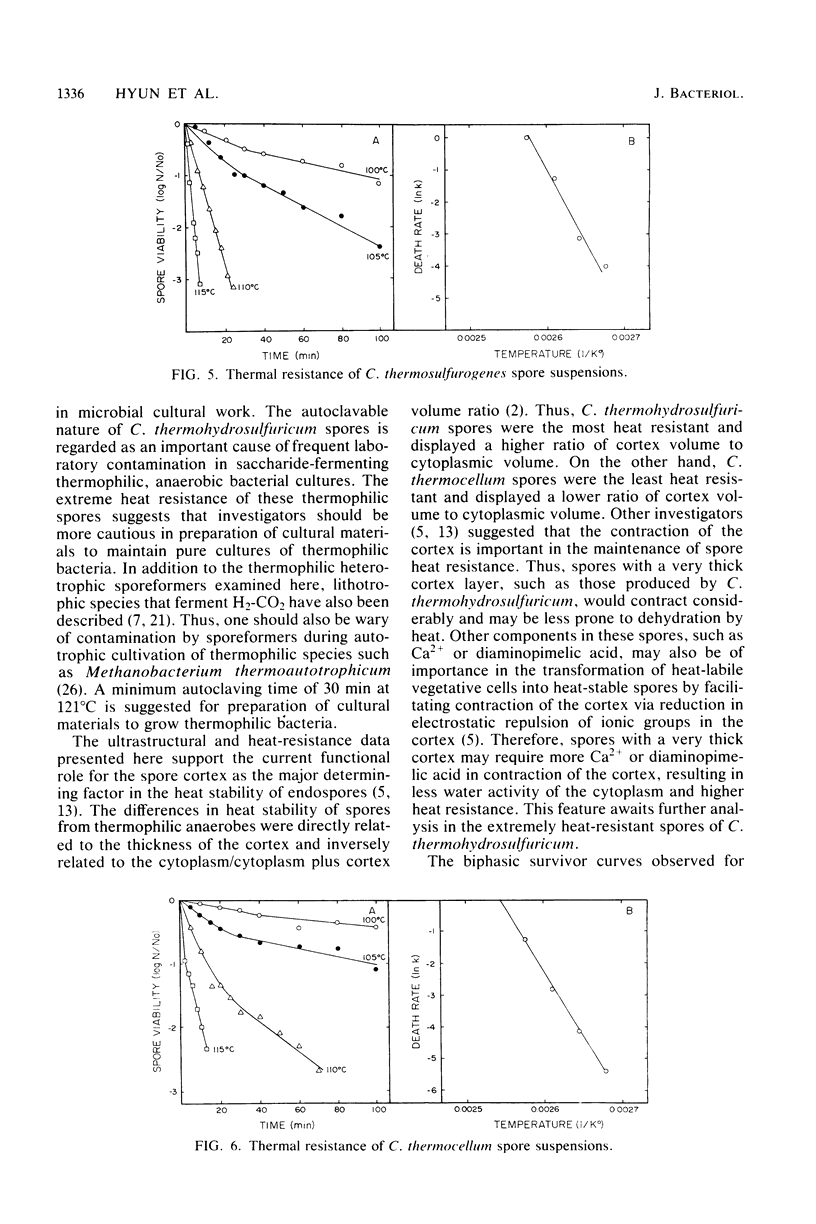
The heat resistance and ultrastructural features of spore suspensions prepared from Clostridium thermocellum LQRI, Clostridium thermosulfurogenes 4B, and Clostridium thermohydrosulfuricum 39E were compared as a function of decimal reduction time. The decimal reduction times at 121 degrees C for strains LQRI, 4B, and 39E were 0.5, 2.5, and 11 min. The higher degree of spore heat resistance was associated with a spore architecture displaying a thicker cortex layer. Heat resistance of these spores was proportional to the ratio of spore cortex volume to cytoplasmic volume. These ratios for spores of strains LQRI, 4B, and 39E were 1.4, 1.6, and 6.6, respectively. The extreme heat resistance and autoclavable nature of C. thermohydrosulfuricum spores under routine sterilization procedures is suggested as a common cause of laboratory contamination with pure cultures of thermophilic, saccharide-fermenting anaerobes.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beaman T. C., Greenamyre J. T., Corner T. R., Pankratz H. S., Gerhardt P. Bacterial spore heat resistance correlated with water content, wet density, and protoplast/sporoplast volume ratio. J Bacteriol. 1982 May;150(2):870–877. doi: 10.1128/jb.150.2.870-877.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donnelly L. S., Busta F. F. Heat resistance of Desulfotomaculum nigrificans spores in soy protein infant formula preparations. Appl Environ Microbiol. 1980 Oct;40(4):721–725. doi: 10.1128/aem.40.4.721-725.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould G. W., Dring G. J. Heat resistance of bacterial endospores and concept of an expanded osmoregulatory cortex. Nature. 1975 Dec 4;258(5534):402–405. doi: 10.1038/258402a0. [DOI] [PubMed] [Google Scholar]
- Han Y. W. Death rates of bacterial spores: nonlinear survivor curves. Can J Microbiol. 1975 Oct;21(10):1464–1467. doi: 10.1139/m75-217. [DOI] [PubMed] [Google Scholar]
- Lamed R., Zeikus J. G. Ethanol production by thermophilic bacteria: relationship between fermentation product yields of and catabolic enzyme activities in Clostridium thermocellum and Thermoanaerobium brockii. J Bacteriol. 1980 Nov;144(2):569–578. doi: 10.1128/jb.144.2.569-578.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millet J., Ryter A. Mutants de Bacillus subtilis Marburg bloqués tardivement dans leur sporulation. Ann Inst Pasteur (Paris) 1972 Mar;122(3):395–406. [PubMed] [Google Scholar]
- Moats W. A. Kinetics of thermal death of bacteria. J Bacteriol. 1971 Jan;105(1):165–171. doi: 10.1128/jb.105.1.165-171.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng T. K., Weimer T. K., Zeikus J. G. Cellulolytic and physiological properties of Clostridium thermocellum. Arch Microbiol. 1977 Jul 26;114(1):1–7. doi: 10.1007/BF00429622. [DOI] [PubMed] [Google Scholar]
- Petre J., Longin R., Millet J. Purification and properties of an endo-beta-1,4-glucanase from Clostridium thermocellum. Biochimie. 1981 Jul;63(7):629–639. doi: 10.1016/s0300-9084(81)80061-2. [DOI] [PubMed] [Google Scholar]
- RYTER A., KELLENBERGER E., BIRCHANDERSEN A., MAALOE O. Etude au microscope électronique de plasmas contenant de l'acide désoxyribonucliéique. I. Les nucléoides des bactéries en croissance active. Z Naturforsch B. 1958 Sep;13B(9):597–605. [PubMed] [Google Scholar]
- Warth A. D. Relationship between the heat resistance of spores and the optimum and maximum growth temperatures of Bacillus species. J Bacteriol. 1978 Jun;134(3):699–705. doi: 10.1128/jb.134.3.699-705.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeikus J. G., Ben-Bassat A., Hegge P. W. Microbiology of methanogenesis in thermal, volcanic environments. J Bacteriol. 1980 Jul;143(1):432–440. doi: 10.1128/jb.143.1.432-440.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeikus J. G., Wolfe R. S. Methanobacterium thermoautotrophicus sp. n., an anaerobic, autotrophic, extreme thermophile. J Bacteriol. 1972 Feb;109(2):707–715. doi: 10.1128/jb.109.2.707-713.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]