Abstract
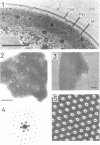
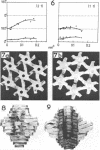
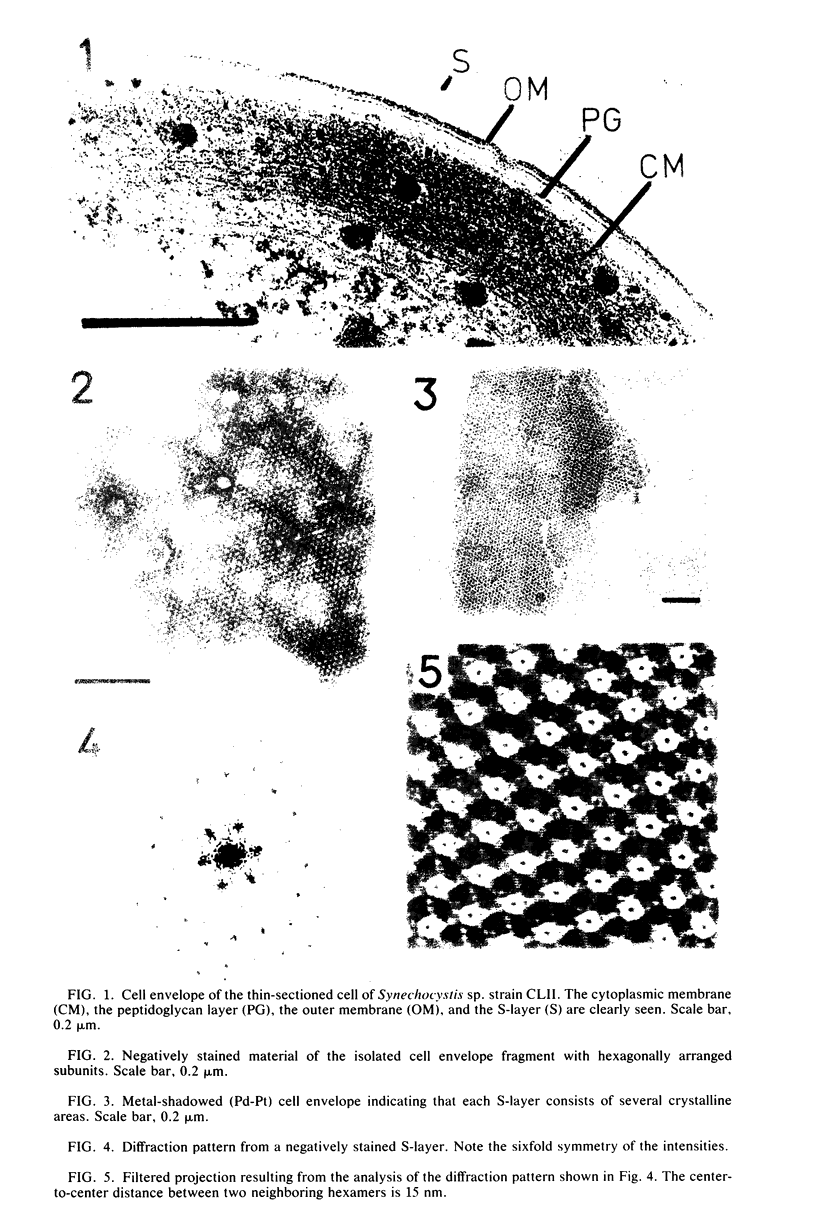
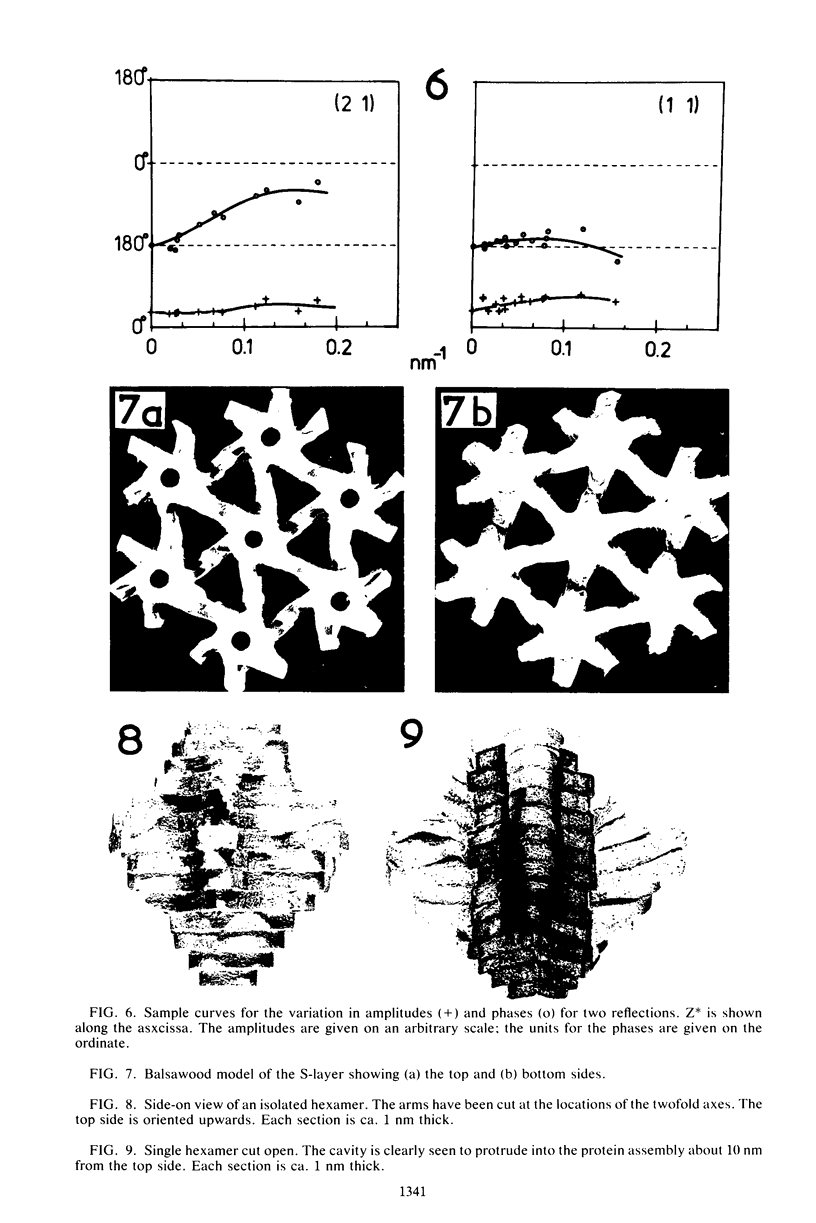
The isolated, outermost cell wall layer from Synechocystis sp. strain CLII is described using electron microscopy and Fourier reconstruction to study the three-dimensional structure of the proteins within the layer to a resolution of ca. 3 nm. This surface layer forms regular hexagonal arrays (a = b = 15.2 nm). The two-dimensional space group is p6. The monomer proteins form hexamers arranged around a central hollow cylinder. The linkers between the hexamers are of the delta type and are located approximately in the central section between the top and bottom of the protein layer.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beveridge T. J. Bacterial structure and its implications in the mechanisms of infection: a short review. Can J Microbiol. 1980 Jun;26(6):643–653. doi: 10.1139/m80-113. [DOI] [PubMed] [Google Scholar]
- Beveridge T. J., Murray R. G. Dependence of the superficial layers of Spirillum putridiconchylium on Ca2+ or Sr2+. Can J Microbiol. 1976 Sep;22(9):1233–1244. doi: 10.1139/m76-183. [DOI] [PubMed] [Google Scholar]
- Beveridge T. J., Murray R. G. Reassembly in vitro of the superficial cell wall components of Spirillum putridiconcyhylium. J Ultrastruct Res. 1976 Apr;55(1):105–118. doi: 10.1016/s0022-5320(76)80086-x. [DOI] [PubMed] [Google Scholar]
- Beveridge T. J., Murray R. G. Superficial macromolecular arrays on the cell wall of Spirillum putridiconchylium. J Bacteriol. 1974 Sep;119(3):1019–1038. doi: 10.1128/jb.119.3.1019-1038.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beveridge T. J. Surface arrays on the wall of Sporosarcina ureae. J Bacteriol. 1979 Sep;139(3):1039–1048. doi: 10.1128/jb.139.3.1039-1048.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beveridge T. J. Ultrastructure, chemistry, and function of the bacterial wall. Int Rev Cytol. 1981;72:229–317. doi: 10.1016/s0074-7696(08)61198-5. [DOI] [PubMed] [Google Scholar]
- Blaurock A. E., Stoeckenius W., Oesterhelt D., Scherfhof G. L. Structure of the cell envelope of Halobacterium halobium. J Cell Biol. 1976 Oct;71(1):1–22. doi: 10.1083/jcb.71.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckmire F. L., Murray R. G. Studies on the cell wall of Spirillum serpens. 1. Isolation and partial purification of the outermost cell wall layer. Can J Microbiol. 1970 Oct;16(10):1011–1022. doi: 10.1139/m70-171. [DOI] [PubMed] [Google Scholar]
- Buckmire F. L., Murray R. G. Studies on the cell wall of Spirillum serpens. II. Chemical characterization of the outer structured layer. Can J Microbiol. 1973 Jan;19(1):59–66. doi: 10.1139/m73-009. [DOI] [PubMed] [Google Scholar]
- Buckmire F. L., Murray R. G. Substructure and in vitro assembly of the outer, structured layer of Spirillum serpens. J Bacteriol. 1976 Jan;125(1):290–299. doi: 10.1128/jb.125.1.290-299.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang J. J., Leonard K., Arad T., Pitt T., Zhang Y. X., Zhang L. H. Structural studies of the outer envelope of Chlamydia trachomatis by electron microscopy. J Mol Biol. 1982 Nov 15;161(4):579–590. doi: 10.1016/0022-2836(82)90409-0. [DOI] [PubMed] [Google Scholar]
- Glaeser R. M., Chiu W., Grano D. Structure of the surface layer protein of the outer membrane of Spirillum serpens. J Ultrastruct Res. 1979 Mar;66(3):235–242. doi: 10.1016/s0022-5320(79)90121-7. [DOI] [PubMed] [Google Scholar]
- Hastie A. T., Brinton C. C., Jr Isolation, characterization, and in vitro assembly of the tetragonally arrayed layer of Bacillus sphaericus. J Bacteriol. 1979 Jun;138(3):999–1009. doi: 10.1128/jb.138.3.999-1009.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson R., Unwin P. N. Three-dimensional model of purple membrane obtained by electron microscopy. Nature. 1975 Sep 4;257(5521):28–32. doi: 10.1038/257028a0. [DOI] [PubMed] [Google Scholar]
- Kay W. W., Buckley J. T., Ishiguro E. E., Phipps B. M., Monette J. P., Trust T. J. Purification and disposition of a surface protein associated with virulence of Aeromonas salmonicida. J Bacteriol. 1981 Sep;147(3):1077–1084. doi: 10.1128/jb.147.3.1077-1084.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessel M. A unique crystalline wall layer in the cyanobacterium Microcystis marginata. J Ultrastruct Res. 1978 Mar;62(3):203–212. doi: 10.1016/s0022-5320(78)80017-3. [DOI] [PubMed] [Google Scholar]
- Koval S. F., Murray R. G. Cell wall proteins of Aquaspirillum serpens. J Bacteriol. 1981 Jun;146(3):1083–1090. doi: 10.1128/jb.146.3.1083-1090.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard K., Wingfield P., Arad T., Weiss H. Three-dimensional structure of ubiquinol:cytochrome c reductase from Neurospora mitochondria determined by electron microscopy of membrane crystals. J Mol Biol. 1981 Jun 25;149(2):259–274. doi: 10.1016/0022-2836(81)90301-6. [DOI] [PubMed] [Google Scholar]
- Lounatmaa K., Vaara T., Osterlund K., Vaara M. Ultrastructure of the cell wall of a Synechocystis strain. Can J Microbiol. 1980 Feb;26(2):204–208. doi: 10.1139/m80-031. [DOI] [PubMed] [Google Scholar]
- Luft J. H. Ruthenium red and violet. I. Chemistry, purification, methods of use for electron microscopy and mechanism of action. Anat Rec. 1971 Nov;171(3):347–368. doi: 10.1002/ar.1091710302. [DOI] [PubMed] [Google Scholar]
- Saxton W. O., Baumeister W. The correlation averaging of a regularly arranged bacterial cell envelope protein. J Microsc. 1982 Aug;127(Pt 2):127–138. doi: 10.1111/j.1365-2818.1982.tb00405.x. [DOI] [PubMed] [Google Scholar]
- Sleytr U. B. Regular arrays of macromolecules on bacterial cell walls: structure, chemistry, assembly, and function. Int Rev Cytol. 1978;53:1–62. doi: 10.1016/s0074-7696(08)62240-8. [DOI] [PubMed] [Google Scholar]
- Smit J., Grano D. A., Glaeser R. M., Agabian N. Periodic surface array in Caulobacter crescentus: fine structure and chemical analysis. J Bacteriol. 1981 Jun;146(3):1135–1150. doi: 10.1128/jb.146.3.1135-1150.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanier R. Y., Cohen-Bazire G. Phototrophic prokaryotes: the cyanobacteria. Annu Rev Microbiol. 1977;31:225–274. doi: 10.1146/annurev.mi.31.100177.001301. [DOI] [PubMed] [Google Scholar]
- Stanier R. Y., Kunisawa R., Mandel M., Cohen-Bazire G. Purification and properties of unicellular blue-green algae (order Chroococcales). Bacteriol Rev. 1971 Jun;35(2):171–205. doi: 10.1128/br.35.2.171-205.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart M., Beveridge T. J., Murray R. G. Structure of the regular surface layer of Spirillum putridiconchylium. J Mol Biol. 1980 Feb 15;137(1):1–8. doi: 10.1016/0022-2836(80)90153-9. [DOI] [PubMed] [Google Scholar]
- Stewart M., Beveridge T. J. Structure of the regular surface layer of Sporosarcina ureae. J Bacteriol. 1980 Apr;142(1):302–309. doi: 10.1128/jb.142.1.302-309.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart M., Murray R. G. Structure of the regular surface layer of Aquaspirillum serpens MW5. J Bacteriol. 1982 Apr;150(1):348–357. doi: 10.1128/jb.150.1.348-357.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaara T., Vaara M., Niemelä S. Two improved methods for obtaining axenic cultures of cyanobacteria. Appl Environ Microbiol. 1979 Nov;38(5):1011–1014. doi: 10.1128/aem.38.5.1011-1014.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss R. L. Subunit cell wall of Sulfolobus acidocaldarius. J Bacteriol. 1974 Apr;118(1):275–284. doi: 10.1128/jb.118.1.275-284.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]