Abstract
Ever since x-rays were shown to induce mutation in Drosophila more than 70 years ago, prevailing dogma considered the genotoxic effects of ionizing radiation, such as mutations and carcinogenesis, as being due mostly to direct damage to the nucleus. Although there was indication that alpha particle traversal through cellular cytoplasm was innocuous, the full impact remained unknown. The availability of the microbeam at the Radiological Research Accelerator Facility of Columbia University made it possible to target and irradiate the cytoplasm of individual cells in a highly localized spatial region. By using dual fluorochrome dyes (Hoechst and Nile Red) to locate nucleus and cellular cytoplasm, respectively, thereby avoiding inadvertent traversal of nuclei, we show here that cytoplasmic irradiation is mutagenic at the CD59 (S1) locus of human–hamster hybrid (AL) cells, while inflicting minimal cytotoxicity. The principal class of mutations induced are similar to those of spontaneous origin and are entirely different from those of nuclear irradiation. Furthermore, experiments with radical scavenger and inhibitor of intracellular glutathione indicated that the mutagenicity of cytoplasmic irradiation depends on generation of reactive oxygen species. These findings suggest that cytoplasm is an important target for genotoxic effects of ionizing radiation, particularly radon, the second leading cause of lung cancer in the United States. In addition, cytoplasmic traversal by alpha particles may be more dangerous than nuclear traversal, because the mutagenicity is accomplished by little or no killing of the target cells.
Radon is ubiquitous in indoor environments and is recognized as a causal factor for lung cancer, which the U.S. Environmental Protection Agency has estimated accounts for as many as 21,600 cases per year (1). It is a secondary decay product of 238uranium and is a colorless, odorless gas, which decays with a half-life of 3.82 days into a series of short-lived radionuclides that emit high linear energy transfer α-particles (2). To develop a better quantitative assessment of lung cancer risk associated with residential radon exposure, it is essential to derive an understanding of the effects of low dose exposure. Furthermore, understanding radiation carcinogenesis requires information on mechanisms underlying the genotoxic effects of radiation. We showed previously that a single α-particle traversal through the nucleus of the human–hamster hybrid (AL) cells induced a mutant yield that was more than 2-fold above the background level (3). Furthermore, the proportion of mutants with multilocus deletions increased with the number of particle traversals. With improvement in the image analysis system, which permits selective targeting and irradiation of cellular cytoplasm in a way similar to the nuclear irradiation, we are able to test the dogmatic theme that DNA is the quintessential genetic target by examining the genotoxicity of cytoplasmic irradiation in mammalian cells.
Ever since x-rays were shown to induce mutation in Drosophila more than 70 years ago, it has always been assumed that the deleterious effects of ionizing radiation, such as mutation and carcinogenesis, are due mainly to direct damage to DNA. Although evidence suggesting that extracellular/extranuclear targets may play a role in such damage has surfaced recently, direct proof of this has not been available. It was found, for example, that very low doses of α-particles induced clastogenic responses (principally sister chromatid exchanges) in both Chinese hamster ovary (CHO) and human fibroblast cultures at levels significantly higher than expected, based on the number of cells that had been traversed by a particle (4, 5). The additional responding cells, which received no radiation exposure, were “bystanders” of either directly hit cells or resulted from agents released from the irradiated medium (5). Subsequent studies suggested that reactive oxygen species may contribute to the induction of SCE among the bystander cells (6). Enhanced expression of the p53 tumor suppressor gene in bystander cells has also been reported in immortalized rat lung epithelial cells irradiated with α-particles (7). The biological consequences of irradiating cytoplasm are largely unknown. To address this issue, we used a charged particle microbeam (3), where cytoplasm of individual AL human–hamster hybrid cells could be targeted and irradiated with high precision to quantify clonogenic survival and mutations induced by defined numbers of α-particle traversals at 90 keV/μm. Our data demonstrate that irradiation of cytoplasm produces gene mutations in the nucleus and that free radicals mediate the process.
MATERIALS AND METHODS
Cell Culture.
The AL hybrid cells that contain a standard set of CHO-K1 chromosomes and a single copy of human chromosome 11 were used (8). Chromosome 11 contains the CD59 gene (formerly known as M1C1) at 11p13.5, which encodes the CD59 cell surface antigen (also known as the S1 antigen) that renders AL cells sensitive to killing by a specific mAb, E7.1, in the presence of rabbit serum complement (HPR, Denver, PA). Antibody E7.1 was produced from hybridoma culture as described (9, 10). Cells were maintained in Ham F-12 medium supplemented with 8% heat-inactivated fetal bovine serum, 25 μg/ml gentamycin, and 2× normal glycine (2 × 10−4M) at 37°C in a humidified 5% CO2 incubator, and passaged as described (11, 12).
Irradiation Protocols.
The layout and methods for nuclear irradiation using the microbeam have been described (3). For cytoplasmic irradiation, Hoechst 33342 and cytoplasmic Nile Red (13) fluorochromes were used to stain the nucleus and cytoplasm, respectively. An image analysis system with a computer controlled stage was used to position cells with an accuracy of ±1 μm over the collimated particle beam. The filter cube (Omega Optical XF06), which had a 366 nm band-pass illumination, was chosen to maximize contrast and to minimize UV exposure. Cells were viewed with a channel–plate image intensifier and an integrating CDD camera, which provided excellent low light sensitivity, thus allowing low intensity illumination and very low concentrations of fluorochromes. Approximately 300 AL cells were seeded overnight into specially constructed microbeam dishes in medium containing 1 mM dibutyryl cAMP to enhance cell spreading (14). Cells were stained for 30 min with a 50 nM solution of Hoechst 33342, washed with medium, and then stained for 10 min with a 100 ng/ml solution of Nile Red. Two images of the cells were captured. The location of individual nuclei in each cell was determined by optical imaging of the fluorescent staining pattern at 366 nm. The cytoplasmic stain was then visualized by using green light excitation and red emission. The images from the film grabber were superimposed into a 24-bit color image using the “merge channel” capacity of Image Pro Plus. The pixel location of the chosen irradiation points and the microscope stage were combined by the computer to calculate the coordinates necessary to position each of these points over the microbeam for irradiation. We found that the cytoplasm had a strong tendency to be stretched out along the same axis as the nucleus. The aiming points in these cytoplasmic irradiations were chosen to be 8 μm from the ends of the major axis of each nucleus (see Fig. 1). Thus, the cytoplasm of each cell was irradiated at each of these two sites depicted by the small numbered circles next to the nuclei. The control program included provisions to mask out the nuclear regions and thus prevent irradiation when the aiming point from one cell was within a neighboring nucleus. For irradiation, the culture medium from the microbeam dish was removed and the chosen targets of each cell were automatically positioned over the microbeam collimator. An electrostatic shutter on the accelerator was opened and a precise number of α-particles, determined by the detector mounted on the microscope lens, was delivered. The precision of the targeting was determined by Monte Carlo modeling of 4,279 particles through the collimator system, which showed that when four particles were delivered to the cytoplasm (two particles at each end), the probability that the nucleus was accidentally struck by a scattered particle was 0.4%. It took on average 6 sec to locate and irradiate a cell. Four −6,000 irradiated cells were used per group for each experiment. After every cell on a plate had been irradiated, the cells were removed by trypsinization and replated to measure both survival and mutation. More than 98% of the irradiated cells were recovered from each dish. Neither dAMP, Hoechst 33342, or Nile Red, either alone or in combination, affected the survival, mutagenesis, or radiosensitivity of the cells when used under the conditions described in this study (data not shown).
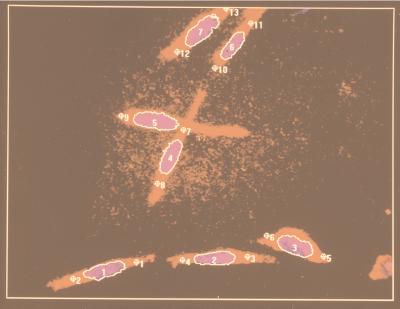
Figure 1.
Dual fluorescent imaging of AL cells stained with Hoechst 33342 (nucleus) and Nile Red (cytoplasm) by the image analysis system under a 40× objective lens. The nucleus of each cell is outlined in white. The image analysis system determines the length of the major axis of each nucleus to calculate the irradiation positions that are chosen to be 8 μm from each end of the nucleus, as shown by the small, numbered circles.
Mutagenesis Assay and Mutant Spectrum Analysis.
Mutation was measured as described (3, 11, 12, 15). Briefly, cells were plated into 60 mm dishes with a total of 2 ml of F12 medium. After 2 hr of incubation to allow for cell attachment, 0.2% CD59 antiserum and 1.5% freshly thawed complement (vol/vol), were added to each dish. The cultures were incubated for 7–8 days, at which time they were fixed, stained, and the number of CD59− mutants scored. Controls included identical sets of dishes containing antiserum alone, complement alone, or neither agent. The mutant fraction at each dose (MF) was calculated as the number of surviving colonies divided by the total number of cells plated after correction for any nonspecific killing due to complement alone.
CD59− mutants were isolated by cloning and expanded in cultures as described (3, 12). Mutational spectra were assessed by using multiplex PCR and primer sequences for five marker genes located on either the short arm (WT, PTH, CAT, RAS) or the long arm (APO-A1) of human chromosome 11. PCR amplifications were performed for 30 cycles as described (3, 12, 15). After the last cycle, the samples were incubated at 72°C for an additional 20 min, electrophoresed on 2% agarose gels, and stained with ethidium bromide.
Treatment with Dimethyl Sulfoxide (DMSO).
To examine the role of reactive oxygen species (ROS) in mediating the mutagenic response to cytoplasmic irradiation, AL cells were treated with 8% DMSO 10 min before and 10 min after irradiation with four α-particle traversals through the cytoplasm. This dose is nontoxic and nonmutagenic under the conditions used in our study and as shown by others (16). After treatment, cells were trypsinized and replated to determine both the survival and mutagenesis as described.
Nonprotein Sulfhydryl Depletion by Buthionine-S-R-Sulfoximine (BSO).
AL cells in microbeam dishes were treated with a 10 μM, nontoxic and nonmutagenic, dose of BSO (Chemalog) for 18 hr, which reduced the nonprotein sulfhydryl level to less than 5% of the control level based on Tietze’s assay (17, 18). Cells were then irradiated with four α-particles through the cytoplasm. After radiation, cultures were trypsinized and replated for both survival and mutagenesis as described above.
Immunoperoxidase Staining for 8-Hydroxy-Deoxyguanosine (8-OHdG).
8-OHdG is recognized as a reliable marker for oxidative DNA damage in mammalian cells (19, 20). Induction of 8-OHdG in the nucleus of AL cells irradiated with eight α-particles through the cytoplasm was quantified by using the mAb IF7 specific for 8-OHdG coupled with immunoperoxidase staining and an image analysis software as described (20). Briefly, irradiated cells on polypropylene dishes were fixed with cold 5% acid alcohol. Cells were treated with RNase (100 μg/ml) in Tris buffer followed by a 5 min treatment with proteinase K (10 μg/ml) at room temperature. After DNA was denatured using 4N HCl for 5 min at room temperature, cells were treated with 10% normal goat serum in Tris buffer to block nonspecific binding. Cells were stained with the primary antibody (IF7) at 1:30 dilution in 2% BSA overnight at 4°C. After washing, mouse ABC reagent (Vector Laboratories), avidin conjugated to horseradish peroxidase, was added for 20 min followed by treatment with 2-aminobenzidine to visualize the reaction as described (20, 21). A Cell Analysis System 200 microscope (Becton Dickinson) and a cell measurement software package were used to quantify the relative staining intensity from 50 randomly selected cells per dish. A total of 150–200 cells were measured from either the control or the irradiated group.
RESULTS
Cytoplasmic Targeting with the Microbeam.
In determining the biological effects of cytoplasmic irradiation, it is critically important to avoid hitting the nucleus. Fig. 1 shows the fluorescent image of a representative population of AL cells stained with Hoechst 33342 (nucleus) and Nile Red (cytoplasm) as seen by the image analysis system under a 40× objective lens. The aiming points in cytoplasmic irradiation were chosen to be 8 μm from the ends of the long axis of each nucleus (Fig. 1). The cytoplasm of each cell was then irradiated at two separate sites with one-half of the number of particles delivered to each end of the cell. The efficiency of targeting and particle delivery was assessed by viewing each of 250 AL cells with two α-particles (one particle in each end of the cell) and determine the actual placement of particle hits. We found that 75% of the cells had a hit in both selected sites, 15% at one end, and 10.3% of the cells had been missed. In no case was the target within the nuclei of any cells (Fig. 1). AL cells stained with both fluorochromes and mock irradiated had a plating efficiency and background mutant yield comparable to controls.
Lethality and Mutagenicity of Cytoplasmic Irradiation.
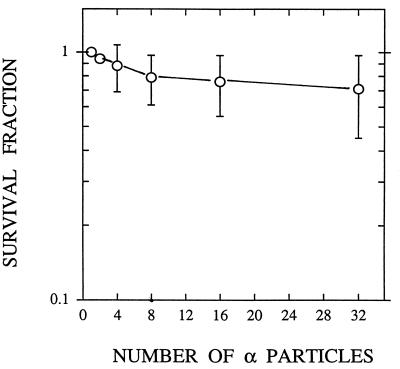
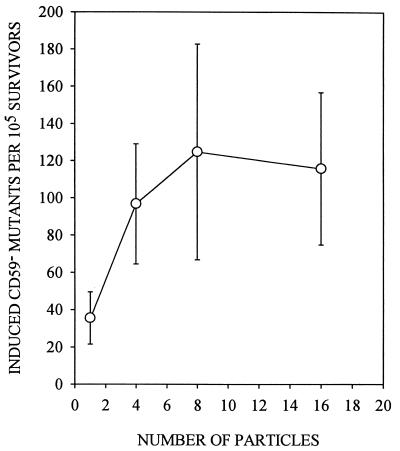
Cytoplasmic irradiation induces minimal toxicity in AL cells, as shown in Fig. 2, such that traversal of cells by four particles results in a surviving fraction of ≈0.9, and more than 70% of the cells survive to form colonies when traversed by 32 α-particles. By comparison, survival after an equivalent number of nuclear traversals was 0.35 and <0.01, respectively (3). By using anti-CD59 antibody that kills wild-type cells in the presence of complement, mutations at the CD59 locus can be quantified. The preexisting level of CD59− mutations was 43 ± 15 mutants per 105 survivors among the AL cell population used in the present studies. Mutant fraction (MF) initially increased with the number of particle traversals, reaching a peak of 125 ± 58 at eight particles, an increase of ≈3-fold over background (Fig. 3). These data indicate that targeted cytoplasm damage can cause mutations in the nucleus. There was, however, no further increase in mutant fraction with particle traversals higher than eight.
Figure 2.
Survival of AL cells irradiated with a single or an exact number of 90 keV/μm α-particles targeted to areas of the cytoplasm. Each data point was obtained from three to seven independent experiments. (Bars represent ± SEM.)
Figure 3.
Induced CD59− mutant fractions per 105 survivors in AL cells irradiated with exact numbers of α-particle traversals through the cytoplasm. Induced mutant frequency equals total mutant yield minus background incidence. Data are pooled from 11 experiments. Induced mutant yield at 32 particles was the same as at eight particles and showed no further increase (data not shown). (Bars represent ± SEM.)
Analysis of Mutant Spectra.
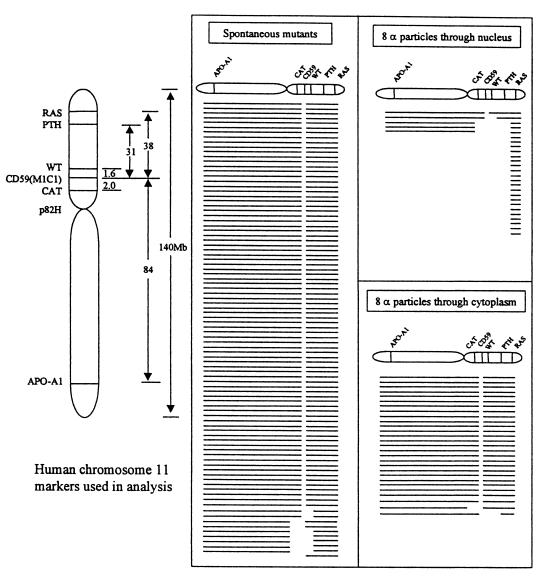
To determine the types and sizes of mutations that caused the CD59− phenotype among AL cell irradiation with α-particles through the cytoplasm, we used multiplex PCR and primer sequences for five marker genes located on either the short arm (Wilm’s tumor, parathyroid hormone, catalase, RAS) or the long arm (apolipoprotein A-1) of human chromosome 11 as described previously (3, 12, 15, 22). These primers and PCR conditions were selected so as to amplify only the human genes and not their CHO cognates (22). Because AL cells have only one chromosome 11, the presence or absence of the corresponding PCR products shows that a particular segment of DNA containing these genes is present or missing, respectively (12, 22). Previous studies have shown that a small region of the distal end of human chromosome 11 at 11p15.5 is required for survival of the AL cells (3, 12, 15, 22). The obligate presence of this region, identified here by the RAS probe in all mutants, provides a convenient internal PCR control. As shown in Fig. 4, most of the mutants induced by eight α-particles through the cytoplasm (four through each end of the cells) had deletion patterns similar to spontaneous mutants that consisted of small alterations involving only the CD59 gene (26/28 or 93% compared with 82/92 or 89% among spontaneous). In contrast, 19/24 or 80% of the mutants induced by an equivalent number of eight particles through nuclei were multilocus deletions (Fig. 4, lower right). The difference in mutant spectra induced by nuclear versus cytoplasmic irradiation suggests that different mutagenic mechanisms are involved.
Figure 4.
Mutational spectra of CD59− mutants either of spontaneous origin or from cells exposed to eight α-particles delivered either to the cytoplasm or the nucleus. Each line represents the spectrum for a single, independent mutant. Blank spaces depict missing markers. The absence or presence of marker genes in each mutant was determined by multiplex PCR. Nuclear irradiation with eight α-particles resulted in 384.6 ± 116 mutants per 105 survivors at a surviving fraction of 12% (3).
Mutagenicity of Cytoplasmic Irradiation Is Mediated by ROS.
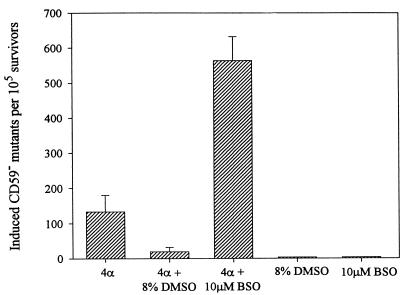
The possible role of ROS in mediating the mutagenesis induced by cytoplasmic irradiation was investigated with two complementary approaches: (i) using the antioxidant DMSO to reduce ROS and (ii) using the thiol-depleting drug BSO to reduce intracellular glutathione. DMSO has been shown to protect against the lethal (23) and genotoxic effects (24, 25) of ionizing radiation in mammalian cells. As shown in Fig. 5, treatment of AL cells with 8% DMSO for 10 min before and 10 min after irradiation with four α-particles significantly suppressed mutation induction by 4- to 5-fold to near background levels. In contrast, pretreatment of AL cells with a 10 μM dose of BSO for 18 hr, which reduced the intracellular glutathione content to <5% of control levels (data not shown), increased the mutagenicity of cytoplasmic irradiation (four α-particles) by 4- to 5-fold. The doses of both the DMSO and BSO used here have been shown to be nontoxic and nonmutagenic in mammalian cells (15–17). These results strongly implicate reactive oxygen species as being the mediator of the mutagenic response of cytoplasmic irradiation. On the other hand, we found that DMSO treatment had no effect on the mutagenic yield in AL cells traversed by four α-particles through nuclei (data not shown).
Figure 5.
Effects of the free radical scavenger DMSO and the thiol-depleting drug BSO on induced mutant yield in AL cells irradiated with four α-particles through the cytoplasm. Cells were treated with 8% DMSO for 10 min before and 10 min after irradiation or with 10 μm BSO for at least 18 hr before irradiation. Data were pooled from three to six experiments. Neither DMSO nor BSO was mutagenic alone. (Bars represent ± SEM.)
Detection of 8-OHdG in Nuclei of Cytoplasmic Irradiated Cells.
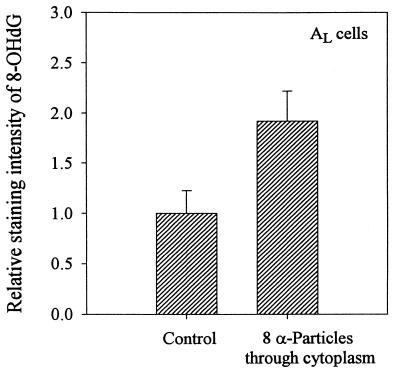
Fig. 6 shows the relative staining intensity of 8-OHdG in control AL cells and those irradiated with eight α-particles targeted to the cytoplasm. The mean background intensity among control cells was 0.051 ± 0.01, whereas the irradiated cells showed a staining intensity of 0.096 ± 0.012, or 1.9-fold higher. The peak expression level of 8-OHdG among irradiated cells was obtained when cells were fixed at 5 min after irradiation. Thereafter, the level decreased rapidly and by 45 min after irradiation, the 8-OHdG level was down to control level (data not shown). These data indicate that cytoplasmic irradiation generates oxidative DNA damages in the nuclei of the target cells.
Figure 6.
Relative immunoperoxidase staining intensity for 8-OHdG in control and AL cells irradiated with eight α-particles through the cytoplasm. Data are averaged from three independent experiments with 60–90 nuclei each. (Bars represent ± SEM.)
DISCUSSION
The differential biological effects of nuclear versus cytoplasmic irradiation has been of interest to biologists and geneticists for decades. Earlier studies using polonium-tipped microneedles to deliver α-particles largely to either the nucleus or cytoplasm of CHO cells showed that the nucleus was the determining site for cellular survival (26), as well as induction of mitotic delay (27). Although these earlier studies were not very precise and used the distance between the needle tip and cell surface to estimate the particle fluency and, derivatively, the dose, nevertheless, they demonstrated that irradiation of cytoplasm was largely innocuous (26) and strongly indicated that DNA was the target for the radiobiological effects of ionizing radiation (28). Recent circumstantial evidence, however, suggests that extranuclear or extracellular targets may also be important in mediating these effects (4–7). In CHO cells irradiated with low doses of α-particles, where <1% of the cells were actually traversed by a particle, an increase in sister chromatid exchanges was observed in more than 30% of the cells (4). Subsequently, based on microdosimetric analysis, it was estimated that the potential target size for this SCE-inducing effect would require an area 350 times the typical size of a CHO nucleus (5). In another words, given the relatively small cytoplasmic area of CHO cells, it is likely that an extracellular component may modulate the observed genotoxic response (5). However, direct proof of such extranuclear/extracellular effects is not available. By using a precision charged particle microbeam, we show here that irradiation of cellular cytoplasm with either a single or an exact number of α-particles results in gene mutation in the nucleus while inflicting minimal toxicity.
Our finding that cytoplasmic irradiation is largely nonlethal is consistent with the earlier reports of Munro (26) and Puck (28). Our observation that the surviving and mutant fractions level off at eight or more particle traversals (Figs. 2 and 3) may reflect that the production of the mediators of mutation was saturated, because only two areas of the cytoplasm were irradiated in each cell. A recent report by Narayanan et al. (6) provides support for this assumption by showing that the intracellular production of O2− in normal human skin fibroblasts irradiated with α-particles was not a function of dose. Alternatively, our results may indicate the induction of a cellular repair process that acts to limit killing and mutations (29). A similar saturating effect has been reported with SCE induction by low doses of alpha particles in CHO cells (5).
It is likely that mutation induced by nuclear traversal is principally a consequence of direct DNA interaction with α-particles, whereas the biological effects of nonnuclear traversals are by indirect action possibly mediated by ROS. This conclusion is supported by the mutant spectra data shown in Fig. 4, where we analyzed the types and size of the CD59− mutants generated by an equivalent number of α-particles targeted either at the nuclear or cytoplasm of the cells. The spectra for cytoplasmic irradiation resemble that found spontaneously, which are often thought to arise as a result of DNA damage from endogenous ROS (30). The clear difference in mutant spectra suggested that different mechanisms are involved in the induction of the mutants at the two target sites.
Our results with the thiol-depleting drug BSO provide further support of the idea that ROS modulate the mutagenic response of cytoplasmic irradiation. BSO, a competitive inhibitor of the enzyme γ-glutamyl cysteine synthetase functions to deplete the intracellular level of nonprotein sulfhydryls, which consist mainly of glutathione (≈95%) and other low molecular weight aminothiols such as cysteine and cysteamine (31). These sulfhydryls have been shown to have significant free radical scavenging abilities that contribute to the maintenance of genomic integrity. Although a decrease in the level of the cellular glutathione is not lethal, it has been shown to enhance the cytotoxicity of a variety of agents, including ionizing radiation and heavy metals (32). Our findings that AL cells in which the level of intracellular nonprotein sulfhydryls has been greatly reduced by BSO treatment showed a 4- to 5-fold increase in mutagenic response to cytoplasmic irradiation compared with similarly irradiated control cultures supports the role of ROS in mediating mutagenicity of cytoplasmic damage. Furthermore, the induction of 8-OHdG in irradiated cells is consistent with a role of oxidative DNA damage.
It is of interest to consider the nature of the events initiated by cytoplasmic irradiation as to what types of oxyradicals are involved and how the signal(s) is transposed from the cytoplasmic target sites to the nucleus where mutagenesis occurs. Because DMSO is a well-established free radical scavenger, particularly of hydroxyl radicals (33), one would expect OH⋅ to be an integral part of the initiating signal. However, OH⋅ is short-lived and can only diffuse ≈4 nm (34), whereas our irradiation sites were ≈8 μm from the nucleus. One possible scenario is that free radicals generated by cytoplasmic irradiation may perpetuate in a cascading event involving lipid peroxidation. Alternatively, organic radicals such as peroxynitrite anions generated as a result of mitochondrial damage could also be involved (35, 36). There is evidence that mitochondrial DNA damage may also modulate DNA damage, although the exact mechanism of how mitochondrial DNA escapes into the nuclear compartment is not known (37).
Finally, although nuclear irradiation induced 3- to 4-fold more CD59− mutants than cytoplasmic irradiation at equivalent particle traversals, the latter is more important to carcinogenesis because it induces mutants with little or no killing. For example, at an equitoxic dose level (e.g., 90% survival), cytoplasmic irradiation induced 7-fold more mutants than nuclear irradiation (3). Therefore, cytoplasmic irradiation should be considered a major concern to human health in terms of risk of exposure for cancer and birth defects, as well as having a profound impact on our understanding of the relationship between radiation exposure and disease.
Acknowledgments
This work was part of a Ph.D. thesis study by L. J. Wu. We thank our Columbia University colleague Dr. Regina Santella for the IF7 mAb. This work was supported by National Institutes of Health Grants CA49062, CA75384, CA36447, and CA56392, National Institutes of Health Research Resource Center Grant RR11623, and National Aeronautics and Space Administration Contract NAF 9501-0232 and NASA-NSCORT. C.W. is a member of the Cancer Center, University of Colorado Health Sciences Center.
ABBREVIATIONS
- CHO
Chinese hamster ovary
- DMSO
dimethyl sulfoxide
- ROS
reactive oxygen species
- BSO
buthionine-S-R-sulfoximine
- 8-OHdG
8-hydroxy-deoxyguanosine
Footnotes
This paper was submitted directly (Track II) to the Proceedings Office.
References
- 1.Environmental Protection Agency. Technical Support Document for Citizen’s Guide to Radon EPA 400-R-92. Washington, DC: U.S. Environmental Protection Agency; 1992. [Google Scholar]
- 2.National Council on Radiation Protection and Measurements. National Council on Radiation Protection and Measurements Report No. 79. Washington, DC: Natl. Acad. Sci.; 1984. [Google Scholar]
- 3.Hei T K, Wu L J, Liu S X, Vannais D, Waldren C A. Proc Natl Acad Sci USA. 1997;94:3765–3770. doi: 10.1073/pnas.94.8.3765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nagasawa H, Little J B. Cancer Res. 1992;52:6394–6396. [PubMed] [Google Scholar]
- 5.Deshpande A, Goodwin E H, Bailey S M, Marrone B L, Lehnert B E. Radiat Res. 1996;145:260–267. [PubMed] [Google Scholar]
- 6.Narayanan P K, Goodwin E H, Lehnert B E. Cancer Res. 1997;57:3963–3971. [PubMed] [Google Scholar]
- 7.Hickman A W, Jaramillo R J, Lechner J F, Johnson N F. Cancer Res. 1994;54:5797–5800. [PubMed] [Google Scholar]
- 8.Puck T T, Wuchier P, Jones C, Kao F T. Proc Natl Acad Sci USA. 1971;68:3102–3106. doi: 10.1073/pnas.68.12.3102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Waldren C, Correll L, Sognier M A, Puck T T. Proc Natl Acad Sci USA. 1986;83:4839–4843. doi: 10.1073/pnas.83.13.4839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vannais D, White M, McGraw M, Davies A, Wilson A, Hei T, Waldren C. Proc Radiat Res Soc Meeting. 1998;P:04–63. , 106 (abstr.). [Google Scholar]
- 11.Hei T K, Piao C Q, Zhu Y-H, Vannais D, Waldren C A. Cancer Res. 1992;52:6305–6309. [PubMed] [Google Scholar]
- 12.Zhu L X, Waldren C A, Vannais D, Hei T K. Radiat Res. 1996;145:251–259. [PubMed] [Google Scholar]
- 13.Greenspan P, Mayer E P, Fowler S D. J Cell Biol. 1985;100:965–973. doi: 10.1083/jcb.100.3.965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Puck T T, Waldren C A, Hsie A W. Proc Natl Acad Sci USA. 1972;69:1943–1947. doi: 10.1073/pnas.69.7.1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hei T K, Liu L X, Waldren C A. Proc Natl Acad Sci USA. 1998;95:8103–8107. doi: 10.1073/pnas.95.14.8103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Watanabe M, Suzuki M, Suzuki K, Hayakawa Y, Miyazaki T. Radiat Res. 1990;124:73–78. [PubMed] [Google Scholar]
- 17.Hei T K, Geard C R, Hall E J. Int J Radiat Oncol Biol Phys. 1984;10:1255–1258. doi: 10.1016/0360-3016(84)90328-6. [DOI] [PubMed] [Google Scholar]
- 18.Tietze F. Anal Biochem. 1969;27:505–509. doi: 10.1016/0003-2697(69)90064-5. [DOI] [PubMed] [Google Scholar]
- 19.Ames B M. Free Radical Res Commun. 1989;7:121–128. doi: 10.3109/10715768909087933. [DOI] [PubMed] [Google Scholar]
- 20.Yarborough A, Zhang Y J, Hsu T M, Santella R. Cancer Res. 1996;56:683–688. [PubMed] [Google Scholar]
- 21.Hei T K, Piao C Q, Sutter T, Willey J C, Suzuki K. Adv Space Res. 1996;18:37–148. doi: 10.1016/0273-1177(95)00800-t. [DOI] [PubMed] [Google Scholar]
- 22.McGuinness S M, Shibuya S M, Ueno A M, Vannais D, Waldren C A. Radiat Res. 1995;142:247–255. [PubMed] [Google Scholar]
- 23.Chapman J D. Radiat Environ Biophys. 1979;16:29–41. doi: 10.1007/BF01326894. [DOI] [PubMed] [Google Scholar]
- 24.Okada S, Nakamura N, Sakai K. In: Radioprotectors and Anticarcinogenesis. Nygaard O F, Simic M G, editors. New York: Academic; 1983. pp. 339–356. [Google Scholar]
- 25.Littlefield L G, Joiner E E, Colyer S P, Sayer A M, Frome E L. Int J Radiat Biol. 1988;53:875–890. doi: 10.1080/09553008814551241. [DOI] [PubMed] [Google Scholar]
- 26.Munro T R. Radiat Res. 1970;42:451–470. [PubMed] [Google Scholar]
- 27.Munro T R. Radiat Res. 1970;44:748–757. [PubMed] [Google Scholar]
- 28.Puck T T. The Mammalian Cell as a Microorganism. San Francisco: Holden-Day; 1972. pp. 102–130. [Google Scholar]
- 29.Samson L, Schwartz J L. Nature (London) 1980;287:861–864. doi: 10.1038/287861a0. [DOI] [PubMed] [Google Scholar]
- 30.Rossman T G, Goncharova E T. Mutat Res. 1998;402:103–110. doi: 10.1016/s0027-5107(97)00287-x. [DOI] [PubMed] [Google Scholar]
- 31.Meister A, Anderson M E. Annu Rev Biochem. 1983;52:711–760. doi: 10.1146/annurev.bi.52.070183.003431. [DOI] [PubMed] [Google Scholar]
- 32.Biaglow J E, Varnes M E, Clark E P, Epp E R. Radiat Res. 1983;95:437–445. [PubMed] [Google Scholar]
- 33.Kennedy A, Symons M C R. Carcinogenesis. 1987;8:683–688. doi: 10.1093/carcin/8.5.683. [DOI] [PubMed] [Google Scholar]
- 34.Roots R, Okada S. Int J Radiat Biol. 1972;21:329–342. doi: 10.1080/09553007214550401. [DOI] [PubMed] [Google Scholar]
- 35.Wei Y H. Proc Soc Exp Biol Med. 1998;217:53–63. doi: 10.3181/00379727-217-44205. [DOI] [PubMed] [Google Scholar]
- 36.Lenaz G. Biochem Biophys Acta. 1998;1366:53–67. doi: 10.1016/s0005-2728(98)00120-0. [DOI] [PubMed] [Google Scholar]
- 37.Cavalli L R, Liang B C. Mutat Res. 1998;398:19–26. doi: 10.1016/s0027-5107(97)00223-6. [DOI] [PubMed] [Google Scholar]